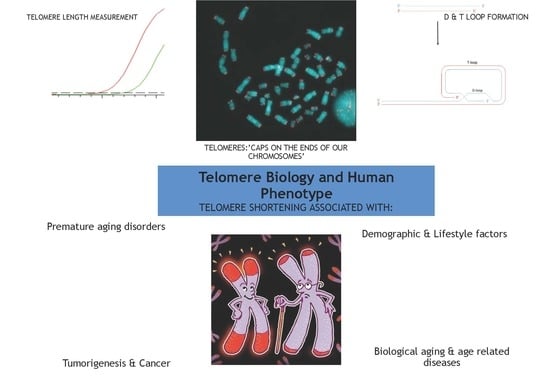
Telomere Biology and Human Phenotype
Abstract
:1. Introduction: Structure, Function and Maintenance of the Telomere
2. Telomere Length and Replicative Capacity
3. Telomere Homeostasis Throughout a Life-Time
Telomere Length in Relation to Demographic and Lifestyle Factors
4. Telomere Length and Biological Aging
4.1. Telomere Biology and Premature Aging Disorders
4.2. Telomere Length in Age-Related Cardiometabolic and Neurological Disorders
4.3. Telomeres, Tumorigenesis and Cancer
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Blackburn, E.; Szostak, J. The molecular structure of centromeres and telomeres. Annu. Rev. Biochem. 1984, 53, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Meyne, J.; Ratliff, R.L.; MoYzIs, R.K. Conservation of the human telomere sequence (TTAGGG) n among vertebrates. Proc. Natl. Acad. Sci. USA 1989, 86, 7049–7053. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. T-loops and the origin of telomeres. Nat. Rev. Mol. Cell Biol. 2004, 5, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.J.; Wu, Y.; Zakian, V.A. DNA repair at telomeres: Keeping the ends intact. Cold Spring Harb. Perspect. Biol. 2013, 5, a012666. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M.; Meyne, J.; Chen, D.J.; Kurimasa, A.; Li, G.C.; Lehnert, B.E.; Goodwin, E.H. DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc. Natl. Acad. Sci. USA 1999, 96, 14899–14904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, S.M.; Murnane, J.P. Telomeres, chromosome instability and cancer. Nucleic Acids Res. 2006, 34, 2408–2417. [Google Scholar] [CrossRef] [Green Version]
- Dechat, T.; Gajewski, A.; Korbei, B.; Gerlich, D.; Daigle, N.; Haraguchi, T.; Furukawa, K.; Ellenberg, J.; Foisner, R. LAP2α and BAF transiently localize to telomeres and specific regions on chromatin during nuclear assembly. J. Cell Sci. 2004, 117, 6117–6128. [Google Scholar] [CrossRef]
- Novo, C.L.; Londono-Vallejo, J.A. Telomeres and the nucleus. Semin. Cancer Biol. 2013, 23, 116–124. [Google Scholar] [CrossRef]
- Blasco, M.A. The epigenetic regulation of mammalian telomeres. Nat. Rev. Genet. 2007, 8, 299. [Google Scholar] [CrossRef]
- Xin, H.; Liu, D.; Songyang, Z. The telosome/shelterin complex and its functions. Genome Biol. 2008, 9, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzerini-Denchi, E.; Sfeir, A. Stop pulling my strings—What telomeres taught us about the DNA damage response. Nat. Rev. Mol. Cell Biol. 2016, 17, 364. [Google Scholar] [CrossRef]
- Lei, M.; Podell, E.R.; Cech, T.R. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat. Struct. Mol. Biol. 2004, 11, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Ohki, R.; Tsurimoto, T.; Ishikawa, F. In vitro reconstitution of the end replication problem. Mol. Cell. Biol. 2001, 21, 5753–5766. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Okazaki, T.; Sakabe, K.; Sugimoto, K.; Sugino, A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc. Natl. Acad. Sci. USA 1968, 59, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Origin of concatemeric T7DNA. Nature 1972, 239, 197–201. [Google Scholar] [CrossRef]
- Ohki, R.; Ishikawa, F. Telomere-bound TRF1 and TRF2 stall the replication fork at telomeric repeats. Nucleic Acids Res. 2004, 32, 1627–1637. [Google Scholar] [CrossRef] [Green Version]
- Greider, C.W.; Blackburn, E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989, 337, 331–337. [Google Scholar] [CrossRef]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric repeat–containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef]
- Redon, S.; Reichenbach, P.; Lingner, J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010, 38, 5797–5806. [Google Scholar] [CrossRef]
- Henson, J.D.; Neumann, A.A.; Yeager, T.R.; Reddel, R.R. Alternative lengthening of telomeres in mammalian cells. Oncogene 2002, 21, 598. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 1992, 89, 10114–10118. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Hayflick, his limit, and cellular ageing. Nat. Rev. Mol. Cell Biol. 2000, 1, 72. [Google Scholar] [CrossRef] [PubMed]
- Hastie, N.D.; Dempster, M.; Dunlop, M.G.; Thompson, A.M.; Green, D.K.; Allshire, R.C. Telomere reduction in human colorectal carcinoma and with ageing. Nature 1990, 346, 866–868. [Google Scholar] [CrossRef]
- Lindsey, J.; McGill, N.I.; Lindsey, L.A.; Green, D.K.; Cooke, H.J. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 1991, 256, 45–48. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Blackburn, E.H.; Shannon, K.M. The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl. Acad. Sci. USA 1998, 95, 5607–5610. [Google Scholar] [CrossRef] [Green Version]
- Zeichner, S.L.; Palumbo, P.; Feng, Y.; Xiao, X.; Gee, D.; Sleasman, J.; Goodenow, M.; Biggar, R.; Dimitrov, D. Rapid telomere shortening in children. Blood 1999, 93, 2824–2830. [Google Scholar]
- Rufer, N.; Brümmendorf, T.H.; Kolvraa, S.; Bischoff, C.; Christensen, K.; Wadsworth, L.; Schulzer, M.; Lansdorp, P.M. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J. Exp. Med. 1999, 190, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Takubo, K.; Nakamura, K.-I.; Izumiyama, N.; Furugori, E.; Sawabe, M.; Arai, T.; Esaki, Y.; Mafune, K.-I.; Kammori, M.; Fujiwara, M. Telomere shortening with aging in human liver. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, B533–B536. [Google Scholar] [CrossRef] [PubMed]
- Takubo, K.; Nakamura, K.-I.; Izumiyama, N.; Sawabe, M.; Arai, T.; Esaki, Y.; Tanaka, Y.; Mafune, K.-I.; Fujiwara, M.; Kammori, M. Telomere shortening with aging in human esophageal mucosa. Age 1999, 22, 95–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansdorp, P.M. Telomeres, stem cells, and hematology. Blood 2008, 111, 1759–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longhese, M.P. DNA damage response at functional and dysfunctional telomeres. Genes Dev. 2008, 22, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Shay, J.W. Telomerase therapeutics: Telomeres recognized as a DNA damage signal. Clin. Cancer Res. 2003, 9, 3521–3525. [Google Scholar]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Galluzzi, L.; Tajeddine, N.; Ortiz, C.; Criollo, A.; Tasdemir, E.; Morselli, E.; Ben Younes, A.; Maiuri, M.C.; Lavandero, S. Senescence, apoptosis or autophagy? Gerontology 2008, 54, 92–99. [Google Scholar] [CrossRef]
- Armanios, M. Telomeres and age-related disease: How telomere biology informs clinical paradigms. J. Clin. Investig. 2013, 123, 996–1002. [Google Scholar] [CrossRef]
- Sanders, J.L.; Newman, A.B. Telomere Length in Epidemiology: A Biomarker of Aging, Age-Related Disease, Both, or Neither? Epidemiol. Rev. 2013, 35, 112–131. [Google Scholar] [CrossRef] [Green Version]
- Von Zglinicki, T.; Martin-Ruiz, C.M. Telomeres as biomarkers for ageing and age-related diseases. Curr. Mol. Med. 2005, 5, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Wong, H.P.; Rai, J.; Hartshorne, G.M. Telomere lengths in human oocytes, cleavage stage embryos and blastocysts. Mol. Hum. Reprod. 2010. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.L.; Jones, E.L.; Mayer, J.F.; Oehninger, S.; Gibbons, W.E.; Lanzendorf, S.E. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol. Hum. Reprod. 2001, 7, 947–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Kong, F.; Luan, Y.; Sun, C.; Wang, J.; Zhang, L.; Jiang, B.; Qi, T.; Zhao, J.; Zheng, C. Differential shortening rate of telomere length in the development of human fetus. Biochem. Biophys. Res. Commun. 2013, 442, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Youngren, K.; Jeanclos, E.; Aviv, H.; Kimura, M.; Stock, J.; Hanna, M.; Skurnick, J.; Bardeguez, A.; Aviv, A. Synchrony in telomere length of the human fetus. Hum. Genet. 1998, 102, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.K.; Bellantuono, I.; Walkinshaw, S.A.; Alfirevic, Z.; Johnston, T.A.; Subhedar, N.V.; Chittick, R.; Swindell, R.; Wynn, R.F. Telomere length dynamics differ in foetal and early post-natal human leukocytes in a longitudinal study. Biogerontology 2009, 10, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Giudice, L.C. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol. Hum. Reprod. 1997, 3, 769–773. [Google Scholar] [CrossRef] [Green Version]
- Vasu, V.; Turner, K.J.; George, S.; Greenall, J.; Slijepcevic, P.; Griffin, D.K. Preterm infants have significantly longer telomeres than their term born counterparts. PLoS ONE 2017, 12, e0180082. [Google Scholar] [CrossRef]
- Friedrich, U.; Schwab, M.; Griese, E.U.; Fritz, P.; Klotz, U. Telomeres in neonates: New insights in fetal hematopoiesis. Pediatr. Res. 2001, 49, 252–256. [Google Scholar] [CrossRef]
- Menon, R.; Yu, J.; Basanta-Henry, P.; Brou, L.; Berga, S.L.; Fortunato, S.J.; Taylor, R.N. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS ONE 2012, 7, e31136. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere length in the newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Akkad, A.; Hastings, R.; Konje, J.C.; Bell, S.C.; Thurston, H.; Williams, B. Telomere length in small-for-gestational-age babies. BJOG 2006, 113, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Factor-Litvak, P.; Susser, E.; Kezios, K.; McKeague, I.; Kark, J.D.; Hoffman, M.; Kimura, M.; Wapner, R.; Aviv, A. Leukocyte telomere length in newborns: Implications for the role of telomeres in human disease. Pediatrics 2016. [Google Scholar] [CrossRef] [PubMed]
- Njajou, O.T.; Cawthon, R.M.; Damcott, C.M.; Wu, S.H.; Ott, S.; Garant, M.J.; Blackburn, E.H.; Mitchell, B.D.; Shuldiner, A.R.; Hsueh, W.C. Telomere length is paternally inherited and is associated with parental lifespan. Proc. Natl. Acad. Sci. USA 2007, 104, 12135–12139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordfjäll, K.; Larefalk, Å.; Lindgren, P.; Holmberg, D.; Roos, G. Telomere length and heredity: Indications of paternal inheritance. Proc. Natl. Acad. Sci. USA 2005, 102, 16374–16378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, U.; Griese, E.-U.; Schwab, M.; Fritz, P.; Thon, K.-P.; Klotz, U. Telomere length in different tissues of elderly patients. Mech. Ageing Dev. 2000, 119, 89–99. [Google Scholar] [CrossRef]
- Daniali, L.; Benetos, A.; Susser, E.; Kark, J.D.; Labat, C.; Kimura, M.; Desai, K.K.; Granick, M.; Aviv, A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 2013, 4, 1597. [Google Scholar] [CrossRef] [Green Version]
- Nordfjäll, K.; Eliasson, M.; Stegmayr, B.; Melander, O.; Nilsson, P.; Roos, G. Telomere length is associated with obesity parameters but with a gender difference. Obesity 2008, 16, 2682–2689. [Google Scholar] [CrossRef]
- Willeit, P.; Willeit, J.; Brandstätter, A.; Ehrlenbach, S.; Mayr, A.; Gasperi, A.; Weger, S.; Oberhollenzer, F.; Reindl, M.; Kronenberg, F. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1649–1656. [Google Scholar] [CrossRef]
- Henckel, E.; Svenson, U.; Nordlund, B.; Berggren Broström, E.; Hedlin, G.; Degerman, S.; Bohlin, K. Telomere length was similar in school-age children with bronchopulmonary dysplasia and allergic asthma. Acta Paediatr. 2018, 107, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A.; Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S. Leukocyte telomere dynamics: Longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol. 2009, 169, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Kark, J.D.; Susser, E.; Kimura, M.; Sinnreich, R.; Chen, W.; Steenstrup, T.; Christensen, K.; Herbig, U.; von Bornemann Hjelmborg, J. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 2013, 12, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrew, T.; Aviv, A.; Falchi, M.; Surdulescu, G.L.; Gardner, J.P.; Lu, X.; Kimura, M.; Kato, B.S.; Valdes, A.M.; Spector, T.D. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am. J. Hum. Genet. 2006, 78, 480–486. [Google Scholar] [CrossRef]
- Codd, V.; Nelson, C.P.; Albrecht, E.; Mangino, M.; Deelen, J.; Buxton, J.L.; Hottenga, J.J.; Fischer, K.; Esko, T.; Surakka, I. Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 2013, 45, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangino, M.; Hwang, S.-J.; Spector, T.D.; Hunt, S.C.; Kimura, M.; Fitzpatrick, A.L.; Christiansen, L.; Petersen, I.; Elbers, C.C.; Harris, T. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum. Mol. Genet. 2012, 21, 5385–5394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjelmborg, J.B.; Dalgård, C.; Möller, S.; Steenstrup, T.; Kimura, M.; Christensen, K.; Kyvik, K.O.; Aviv, A. The heritability of leucocyte telomere length dynamics. J. Med. Genet. 2015, 52, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Kyo, S.; Takakura, M.; Kanaya, T.; Zhuo, W.; Fujimoto, K.; Nishio, Y.; Orimo, A.; Inoue, M. Estrogen activates telomerase. Cancer Res. 1999, 59, 5917–5921. [Google Scholar]
- Simoncini, T.; Hafezi-Moghadam, A.; Brazil, D.P.; Ley, K.; Chin, W.W.; Liao, J.K. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 2000, 407, 538–541. [Google Scholar] [CrossRef]
- Kang, S.S.; Kwon, T.; Do, S.I. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J. Biol. Chem. 1999, 274, 13085–13090. [Google Scholar] [CrossRef]
- Diez Roux, A.V.; Ranjit, N.; Jenny, N.S.; Shea, S.; Cushman, M.; Fitzpatrick, A.; Seeman, T. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell 2009, 8, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Epel, E.S. Psychological and metabolic stress: A recipe for accelerated cellular aging. Hormones 2009, 8, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.M.; Smoller, J.W.; McNamara, K.L.; Maser, R.S.; Zalta, A.K.; Pollack, M.H.; Nierenberg, A.A.; Fava, M.; Wong, K.-K. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry 2006, 60, 432–435. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Mellon, S.H.; Epel, E.S.; Lin, J.; Dhabhar, F.S.; Su, Y.; Reus, V.I.; Rosser, R.; Burke, H.M.; Kupferman, E. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress-preliminary findings. PLoS ONE 2011, 6, e17837. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, L.F.; Hunkin, J.L.; Kato, B.S.; Richards, J.B.; Gardner, J.P.; Surdulescu, G.L.; Kimura, M.; Lu, X.; Spector, T.D.; Aviv, A. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 2008, 168, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Puterman, E.; Lin, J.; Blackburn, E.; O’Donovan, A.; Adler, N.; Epel, E. The power of exercise: Buffering the effect of chronic stress on telomere length. PLoS ONE 2010, 5, e10837. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, A.T.; Zimmerman, J.B.; Witkowski, S.; Hearn, J.W.; Hatfield, B.D.; Roth, S.M. Relationship between physical activity level, telomere length, and telomerase activity. Med. Sci. Sports Exerc. 2008, 40, 1764. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Andrew, T.; Gardner, J.A.; Kimura, M.; Oelsner, E.; Cherkas, L.; Aviv, A.; Spector, T. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Das, U.N. Metabolic syndrome X: An inflammatory condition? Curr. Hypertens. Rep. 2004, 6, 66–73. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [Green Version]
- Carulli, L.; Anzivino, C.; Baldelli, E.; Zenobii, M.; Rocchi, M.B.L.; Bertolotti, M. Telomere length elongation after weight loss intervention in obese adults. Mol. Genet. Metab. 2016, 118, 138–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, M.; Wong, J.Y.; Michaud, D.; Hunter, D.J.; De Vivo, I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol. Biomark. Prev. 2007, 16, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Hoxha, M.; Dioni, L.; Bertazzi, P.A.; Snenghi, R.; Nalesso, A.; Ferrara, S.D.; Montisci, M.; Baccarelli, A. Shortened telomeres in individuals with abuse in alcohol consumption. Int. J. Cancer 2011, 129, 983–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Blasco, M.A.; Serrano, M. Cellular senescence in cancer and aging. Cell 2007, 130, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.F.; Saretzki, G.; von Zglinicki, T. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007, 35, 7505–7513. [Google Scholar] [CrossRef]
- Jenny, N.S. Inflammation in aging: Cause, effect, or both? Discov. Med. 2012, 13, 451–460. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239. [Google Scholar] [CrossRef]
- Oikawa, S.; Kawanishi, S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999, 453, 365–368. [Google Scholar] [CrossRef] [Green Version]
- De Sandre-Giovannoli, A.; Bernard, R.; Cau, P.; Navarro, C.; Amiel, J.; Boccaccio, I.; Lyonnet, S.; Stewart, C.L.; Munnich, A.; Le Merrer, M. Lamin a truncation in Hutchinson-Gilford progeria. Science 2003, 300, 2055. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.L.; Chavez, E.; Vulto, I.; Lansdorp, P.M. Telomere length in Hutchinson-Gilford progeria syndrome. Mech. Ageing Dev. 2009, 130, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.K.; Lee, S.W.; Aaronson, S.A. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J. Cell Sci. 2010, 123, 2605–2612. [Google Scholar] [CrossRef]
- Goto, M.; Rubenstein, M.; Weber, J.; Woods, K.; Drayna, D. Genetic linkage of Werner’s syndrome to five markers on chromosome 8. Nature 1992, 355, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Schulz, V.P.; Zakian, V.A.; Ogburn, C.E.; McKay, J.; Jarzebowicz, A.A.; Martin, G.; Edland, S. Accelerated loss of telomeric repeats may not explain accelerated replicative decline of Werner syndrome cells. Hum. Genet. 1996, 97, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.M.; Davis, T.; Rowson, J.; Jones, C.J.; Kipling, D. Normal telomere erosion rates at the single cell level in Werner syndrome fibroblast cells. Hum. Mol. Genet. 2004, 13, 1515–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opresko, P.L.; Otterlei, M.; Graakjær, J.; Bruheim, P.; Dawut, L.; Kølvraa, S.; May, A.; Seidman, M.M.; Bohr, V.A. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol. Cell 2004, 14, 763–774. [Google Scholar] [CrossRef]
- Crabbe, L.; Jauch, A.; Naeger, C.M.; Holtgreve-Grez, H.; Karlseder, J. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 2205–2210. [Google Scholar] [CrossRef] [Green Version]
- Stavropoulos, D.J.; Bradshaw, P.S.; Li, X.; Pasic, I.; Truong, K.; Ikura, M.; Ungrin, M.; Meyn, M.S. The Bloom syndrome helicase BLM interacts with TRF2 in ALT cells and promotes telomeric DNA synthesis. Hum. Mol. Genet. 2002, 11, 3135–3144. [Google Scholar] [CrossRef] [Green Version]
- Yankiwski, V.; Marciniak, R.A.; Guarente, L.; Neff, N.F. Nuclear structure in normal and Bloom syndrome cells. Proc. Natl. Acad. Sci. USA 2000, 97, 5214–5219. [Google Scholar] [CrossRef] [Green Version]
- German, J. Bloom syndrome: A mendelian prototype of somatic mutational disease. Medicine 1993, 72, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Neff, N.F.; Ellis, N.A.; Ye, T.Z.; Noonan, J.; Huang, K.; Sanz, M.; Proytcheva, M. The DNA helicase activity of BLM is necessary for the correction of the genomic instability of Bloom syndrome cells. Mol. Biol. Cell 1999, 10, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y. Ataxia-telangiectasia and the Nijmegen breakage syndrome: Related disorders but genes apart. Annu. Rev. Genet. 1997, 31, 635–662. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, V.; Heine, W.F.; Ciccone, D.N.; Rudolph, K.L.; Wu, X.; Chang, S.; Hai, H.; Ahearn, I.M.; Livingston, D.M.; Resnick, I. Rescue of a telomere length defect of Nijmegen breakage syndrome cells requires NBS and telomerase catalytic subunit. Curr. Biol. 2001, 11, 962–966. [Google Scholar] [CrossRef] [Green Version]
- Stevnsner, T.; Muftuoglu, M.; Aamann, M.D.; Bohr, V.A. The role of Cockayne Syndrome group B (CSB) protein in base excision repair and aging. Mech. Ageing Dev. 2008, 129, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laugel, V.; Dalloz, C.; Durand, M.; Sauvanaud, F.; Kristensen, U.; Vincent, M.-C.; Pasquier, L.; Odent, S.; Cormier-Daire, V.; Gener, B. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum. Mutat. 2010, 31, 113–126. [Google Scholar] [CrossRef]
- Batenburg, N.L.; Mitchell, T.R.; Leach, D.M.; Rainbow, A.J.; Zhu, X.-D. Cockayne Syndrome group B protein interacts with TRF2 and regulates telomere length and stability. Nucleic Acids Res. 2012, 40, 9661–9674. [Google Scholar] [CrossRef] [Green Version]
- Heiss, N.; Knight, S.; Vulliamy, T.; Klauck, S.; Wiemann, S.; Mason, P.; Poustka, A.; Dokal, I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat. Genet. 1998, 19, 32. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Wood, E.; Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 1999, 402, 551–555. [Google Scholar] [CrossRef]
- Vulliamy, T.; Beswick, R.; Kirwan, M.; Marrone, A.; Digweed, M.; Walne, A.; Dokal, I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc. Natl. Acad. Sci. USA 2008, 105, 8073–8078. [Google Scholar] [CrossRef] [Green Version]
- Savage, S.A.; Giri, N.; Baerlocher, G.M.; Orr, N.; Lansdorp, P.M.; Alter, B.P. TINF2, a Component of the Shelterin Telomere Protection Complex, Is Mutated in Dyskeratosis Congenita. Am. J. Med. Genet. 2008, 82, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walne, A.J.; Vulliamy, T.; Marrone, A.; Beswick, R.; Kirwan, M.; Masunari, Y.; Al-Qurashi, F.-H.; Aljurf, M.; Dokal, I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum. Mol. Genet. 2007, 16, 1619–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savitsky, K.; Bar-Shira, A.; Gilad, S.; Rotman, G.; Ziv, Y.; Vanagaite, L.; Tagle, D.A.; Smith, S.; Uziel, T.; Sfez, S. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 1995, 268, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, J.A.; Parkhill, J.; Campbell, L.; Stacey, M.; Biggs, P.; Byrd, P.J.; Taylor, A.M.R. Accelerated telomere shortening in ataxia telangiectasia. Nat. Genet. 1996, 13, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, H.; Schächter, F.; Uchida, I.; Wei, L.; Zhu, X.; Effros, R.; Cohen, D.; Harley, C. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am. J. Hum. Genet. 1993, 52, 661. [Google Scholar] [PubMed]
- Horvath, S.; Garagnani, P.; Bacalini, M.G.; Pirazzini, C.; Salvioli, S.; Gentilini, D.; Di Blasio, A.M.; Giuliani, C.; Tung, S.; Vinters, H.V. Accelerated epigenetic aging in Down syndrome. Aging Cell 2015, 14, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Head, E.; Lott, I.T.; Wilcock, D.M.; Lemere, C.A. Aging in Down syndrome and the development of Alzheimer’s disease neuropathology. Curr. Alzheimer Res. 2016, 13, 18–29. [Google Scholar] [CrossRef]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kronmal, R.A.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Walston, J.; Kimura, M.; Aviv, A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am. J. Epidemiol. 2007, 165, 14–21. [Google Scholar] [CrossRef]
- Salpea, K.D.; Humphries, S.E. Telomere length in atherosclerosis and diabetes. Atherosclerosis 2010, 209, 35. [Google Scholar] [CrossRef]
- Samani, N.J.; Boultby, R.; Butler, R.; Thompson, J.R.; Goodall, A.H. Telomere shortening in atherosclerosis. Lancet 2001, 358, 472–473. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Khan, M.Y.; Skurnick, J.; Kimura, M.; Aviv, H.; Aviv, A. Telomere attrition of the human abdominal aorta: Relationships with age and atherosclerosis. Atherosclerosis 2000, 152, 391–398. [Google Scholar] [CrossRef]
- Aviv, A.; Aviv, H. Telomeres and essential hypertension. Am. J. Hypertens. 1999, 12, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Von Zglinicki, T.; Serra, V.; Lorenz, M.; Saretzki, G.; Lenzen-Gro, R.; Geβner, R.; Risch, A.; Steinhagen-Thiessen, E. Short telomeres in patients with vascular dementia: An indicator of low antioxidative capacity and a possible risk factor? Lab. Investig. 2000, 80, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chen, A.F.; Wang, H.Z.; Xie, L.Y.; Sui, K.X.; Zhang, Q.Y. Association of shorter mean telomere length with large artery stiffness in patients with coronary heart disease. Aging Male 2011, 14, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kronmal, R.A.; Kimura, M.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Hardikar, S.; Aviv, A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 421–429. [Google Scholar] [CrossRef]
- Epel, E.S.; Merkin, S.S.; Cawthon, R.; Blackburn, E.H.; Adler, N.E.; Pletcher, M.J.; Seeman, T.E. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging 2009, 1, 81. [Google Scholar] [CrossRef]
- Jeanclos, E.; Krolewski, A.; Skurnick, J.; Kimura, M.; Aviv, H.; Warram, J.H.; Aviv, A. Shortened telomere length in white blood cells of patients with IDDM. Diabetes 1998, 47, 482–486. [Google Scholar] [CrossRef]
- Salpea, K.D.; Talmud, P.J.; Cooper, J.A.; Maubaret, C.G.; Stephens, J.W.; Abelak, K.; Humphries, S.E. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 2010, 209, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willeit, P.; Raschenberger, J.; Heydon, E.E.; Tsimikas, S.; Haun, M.; Mayr, A.; Weger, S.; Witztum, J.L.; Butterworth, A.S.; Willeit, J. Leucocyte telomere length and risk of type 2 diabetes mellitus: New prospective cohort study and literature-based meta-analysis. PLoS ONE 2014, 9, e112483. [Google Scholar] [CrossRef] [PubMed]
- Nai-chieh, Y.; Chen, B.H.; Song, Y.; Lu, X.; Chen, Y.; Manson, J.E.; Kang, M.; Howard, B.V.; Margolis, K.L.; Curb, J.D. A prospective study of leukocyte telomere length and risk of type 2 diabetes in postmenopausal women. Diabetes 2012. [Google Scholar] [CrossRef]
- Zhao, J.; Miao, K.; Wang, H.; Ding, H.; Wang, D.W. Association between telomere length and type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2013, 8, e79993. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; O’Callaghan, N.J.; Fenech, M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech. Ageing Dev. 2008, 129, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Panossian, L.; Porter, V.; Valenzuela, H.; Zhu, X.; Reback, E.; Masterman, D.; Cummings, J.; Effros, R. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol. Aging 2003, 24, 77–84. [Google Scholar] [CrossRef]
- Honig, L.S.; Schupf, N.; Lee, J.H.; Tang, M.X.; Mayeux, R. Shorter telomeres are associated with mortality in those with APOE ϵ4 and dementia. Ann. Neurol. 2006, 60, 181–187. [Google Scholar] [CrossRef]
- Grodstein, F.; van Oijen, M.; Irizarry, M.C.; Rosas, H.D.; Hyman, B.T.; Growdon, J.H.; De Vivo, I. Shorter telomeres may mark early risk of dementia: Preliminary analysis of 62 participants from the nurses’ health study. PLoS ONE 2008, 3, e1590. [Google Scholar] [CrossRef]
- Martin-Ruiz, C.; Dickinson, H.O.; Keys, B.; Rowan, E.; Kenny, R.A.; Von Zglinicki, T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann. Neurol. 2006, 60, 174–180. [Google Scholar] [CrossRef]
- Hochstrasser, T.; Marksteiner, J.; Humpel, C. Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Exp. Gerontol. 2012, 47, 160–163. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.; Deary, I.; Gardner, J.; Kimura, M.; Lu, X.; Spector, T.; Aviv, A.; Cherkas, L. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol. Aging 2010, 31, 986–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zekry, D.; Herrmann, F.R.; Irminger-Finger, I.; Ortolan, L.; Genet, C.; Vitale, A.-M.; Michel, J.-P.; Gold, G.; Krause, K.-H. Telomere length is not predictive of dementia or MCI conversion in the oldest old. Neurobiol. Aging 2010, 31, 719–720. [Google Scholar] [CrossRef]
- Watfa, G.; Dragonas, C.; Brosche, T.; Dittrich, R.; Sieber, C.; Alecu, C.; Benetos, A.; Nzietchueng, R. Study of telomere length and different markers of oxidative stress in patients with Parkinson’s disease. J. Nutr. Health Aging 2011, 15, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.Z.; Maeda, T.; Sugano, M.; Oyama, J.-I.; Higuchi, Y.; Suzuki, T.; Makino, N. A percentage analysis of the telomere length in Parkinson’s disease patients. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, H.; Gao, X.; McGrath, M.; Deer, D.; De Vivo, I.; Schwarzschild, M.A.; Ascherio, A. Telomere length and risk of Parkinson’s disease. Mov. Disord. 2008, 23, 302–305. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Andersen, J.K.; Kapahi, P.; Melov, S. Cellular senescence: A link between cancer and age-related degenerative disease? Proce. Semin. Cancer Biol. 2011, 21, 354–359. [Google Scholar] [CrossRef]
- Wright, W.E.; Pereira-Smith, O.; Shay, J. Reversible cellular senescence: Implications for immortalization of normal human diploid fibroblasts. Mol. Cell. Biol. 1989, 9, 3088–3092. [Google Scholar] [CrossRef]
- Oh, B.-K.; Kim, Y.-J.; Park, C.; Park, Y.N. Up-regulation of telomere-binding proteins, TRF1, TRF2, and TIN2 is related to telomere shortening during human multistep hepatocarcinogenesis. Am. J. Pathol. 2005, 166, 73–80. [Google Scholar] [CrossRef]
- De Lange, T. Telomere-related genome instability in cancer. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 197–204. [Google Scholar] [CrossRef]
- Cheung, A.; Deng, W. Telomere dysfunction, genome instability and cancer. Front. Biosci. 2008, 13, 2075–2090. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. Genetic alterations in leukemias and lymphomas: Impressive progress and continuing complexity. Cancer Genet. Cytogenet. 1997, 94, 13–19. [Google Scholar] [CrossRef]
- Albertson, D.G.; Collins, C.; McCormick, F.; Gray, J.W. Chromosome aberrations in solid tumors. Nat. Genet. 2003, 34, 369. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.E.; Shay, J.W. The two-stage mechanism controlling cellular senescence and immortalization. Exp. Gerontol. 1992, 27, 383–389. [Google Scholar] [CrossRef]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaphy, C.M.; Subhawong, A.P.; Hong, S.-M.; Goggins, M.G.; Montgomery, E.A.; Gabrielson, E.; Netto, G.J.; Epstein, J.I.; Lotan, T.L.; Westra, W.H. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am. J. Pathol. 2011, 179, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Tsao, S.W.; Guan, X.Y.; Lucas, J.N.; Cheung, A.L. Role of short telomeres in inducing preferential chromosomal aberrations in human ovarian surface epithelial cells: A combined telomere quantitative fluorescence in situ hybridization and whole-chromosome painting study. Genes Chromosomes Cancer 2003, 37, 92–97. [Google Scholar] [CrossRef]
- Der-Sarkissian, H.; Bacchetti, S.; Cazes, L.; Londoño-Vallejo, J.A. The shortest telomeres drive karyotype evolution in transformed cells. Oncogene 2004, 23, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Chevret, E.; Andrique, L.; Prochazkova-Carlotti, M.; Ferrer, J.; Cappellen, D.; Laharanne, E.; Idrissi, Y.; Boettiger, A.; Sahraoui, W.; Ruiz, F. Telomerase functions beyond telomere maintenance in primary cutaneous T-cell lymphoma. Blood 2014. [Google Scholar] [CrossRef]
- Wentzensen, I.M.; Mirabello, L.; Pfeiffer, R.M.; Savage, S.A. The association of telomere length and cancer: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1238–1250. [Google Scholar] [CrossRef]
- Willeit, P.; Willeit, J.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Brandstätter, A.; Kronenberg, F.; Kiechl, S. Telomere length and risk of incident cancer and cancer mortality. JAMA 2010, 304, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Donaires, F.S.; Scatena, N.F.; Alves-Paiva, R.M.; Podlevsky, J.D.; Logeswaran, D.; Santana, B.A.; Teixeira, A.C.; Chen, J.J.-L.; Calado, R.T.; Martinelli, A.L. Telomere biology and telomerase mutations in cirrhotic patients with hepatocellular carcinoma. PLoS ONE 2017, 12, e0183287. [Google Scholar] [CrossRef] [PubMed]
- Donati, B.; Pietrelli, A.; Pingitore, P.; Dongiovanni, P.; Caddeo, A.; Walker, L.; Baselli, G.; Pelusi, S.; Rosso, C.; Vanni, E. Telomerase reverse transcriptase germline mutations and hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Cancer Med. 2017, 6, 1930–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet. 2017, 49, 349. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; Willeit, P. Association between telomere length and risk of cancer and non-neoplastic diseases: A Mendelian randomization study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef]
- Pellatt, A.J.; Wolff, R.K.; Torres-Mejia, G.; John, E.M.; Herrick, J.S.; Lundgreen, A.; Baumgartner, K.B.; Giuliano, A.R.; Hines, L.M.; Fejerman, L. Telomere length, telomere-related genes, and breast cancer risk: The breast cancer health disparities study. Genes Chromosomes Cancer 2013, 52, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Espiridion, B.; Chen, M.; Chang, J.Y.; Lu, C.; Chang, D.W.; Roth, J.A.; Wu, X.; Gu, J. Telomere length in peripheral blood leukocytes and lung cancer risk: A large case–control study in Caucasians. Cancer Res. 2014. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Z.; Wei, S.; Liu, Z.; Pooley, K.A.; Dunning, A.M.; Svenson, U.; Roos, G.; Hosgood, H.D., III; Shen, M. Shortened telomere length is associated with increased risk of cancer: A meta-analysis. PLoS ONE 2011, 6, e20466. [Google Scholar] [CrossRef]
- Anic, G.M.; Sondak, V.K.; Messina, J.L.; Fenske, N.A.; Zager, J.S.; Cherpelis, B.S.; Lee, J.-H.; Fulp, W.J.; Epling-Burnette, P.K.; Park, J.Y. Telomere length and risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. Cancer Epidemiol. 2013, 37, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Pooley, K.A.; Sandhu, M.S.; Tyrer, J.; Shah, M.; Driver, K.E.; Luben, R.N.; Bingham, S.A.; Ponder, B.A.; Pharoah, P.D.; Khaw, K.-T. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010, 70, 3170–3176. [Google Scholar] [CrossRef]
- Tacutu, R.; Craig, T.; Budovsky, A.; Wuttke, D.; Lehmann, G.; Taranukha, D.; Costa, J.; Fraifeld, V.E.; De Magalhães, J.o.P. Human Ageing Genomic Resources: Integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res. 2012, 41, D1027–D1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dato, S.; Rose, G.; Crocco, P.; Monti, D.; Garagnani, P.; Franceschi, C.; Passarino, G. The genetics of human longevity: An intricacy of genes, environment, culture and microbiome. Mech. Ageing Dev. 2017, 165, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Singer, B.D.; Vaughan, D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 2017, 16, 624–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Ven, R.A.; Santos, D.; Haigis, M.C. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol. Med. 2017, 23, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.J.; Vasu, V.; Greenall, J.; Griffin, D.K. Telomere length analysis and preterm infant health: The importance of assay design in the search for novel biomarkers. Biomark. Med. 2014, 8, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.M.; Johnson, R.A.; Litzelman, K.; Skinner, H.G.; Seo, S.; Engelman, C.D.; Vanderboom, R.J.; Kimmel, G.W.; Gangnon, R.E.; Riegert-Johnson, D.L. Telomere length varies by DNA extraction method: Implications for epidemiologic research. Cancer Epidemiol. Biomark. Prev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zanet, D.L.; Saberi, S.; Oliveira, L.; Sattha, B.; Gadawski, I.; Côté, H.C. Blood and dried blood spot telomere length measurement by qPCR: Assay considerations. PLoS ONE 2013, 8, e57787. [Google Scholar] [CrossRef]

| Protein Name | Interactions | Function |
|---|---|---|
| Telomere repeat binding factor 1 (TERF1 also known as TRF1) | Direct interaction with double stranded TTAGGG repeats | Regulation of telomere length |
| Telomere repeat binding factor 2 (TERF2 also known as TRF2) | Direct interaction with double stranded TTAGGG repeats | Stabilisation of the T-loop and regulation of telomere length |
| TERF1 interacting nuclear factor 2 (TINF2 also known as TIN2) | Associates directly with TERF1, TERF2 and ACD and indirectly with POT1 | Tethering of ACD and POT1 to TERF1 and TERF2 and tethering TERF1 to TERF2, which stabilises the association of TERF2 with the telomere. Also regulates telomere length |
| Protection of Telomeres 1 (POT1) | Direct interaction with single stranded telomere overhang | Inhibition of DNA damage response and regulation of telomere length |
| Shelterin complex subunit and telomerase recruitment factor (ACD, also known as TPP1) | Interaction with TINF2 and POT1 | Enhances POT1 binding to single stranded telomere DNA and regulates telomere length in combination with POT1 |
| TERF2 interacting protein (TERF2IP also known as RAP1) | Associates with TERF2 | Telomere length regulation |
| Demographic Factors | General Observations | References |
|---|---|---|
| Genetic factors | Several twin studies have identified high heritability of telomere length and many specific loci associated with telomere length have been reported. | [64,65,66,67] |
| Gender | Longer telomeres are found in adult females compared to males. This is thought to be due to higher levels of oestrogen, which confers anti-inflammatory as well as antioxidant properties and is known to promote telomerase expression. | [60,68,69,70] |
| Ethnicity | Telomeres are slightly longer in white individuals compared to black and Hispanic individuals. However, this difference is often not statistically significant unless also adjusted for other factors such as age, sex, socio-economic background and lifestyle factors (diet and smoking) | [71] |
| Level of psychosocial stress | Shortened telomeres are associated with high levels of psychosocial stress as a result of increased oxidative stress as well as reduced telomerase activity. Telomere length is also inversely correlated with major depressive disorder due to increased inflammatory factors leading to increased oxidative stress. | [72,73,74] |
| Level of physical activity | Longer telomeres have been found in those that engage in higher levels of physical activity, which is associated with improved physical and psychological wellbeing. Thus it is possible that the effects of physical activity on telomere length are influenced by a positive effect on physical and mental well-being | [75,76,77] |
| Obesity | Telomeres are known to be shortened in obese individuals. Obesity is associated with chronic inflammation, increased reactive oxygen species (ROS) production in adipose tissue and evidence of increased systemic oxidative stress. Furthermore, telomere length is correlated with body mass index (BMI), with increased BMI resulting in higher blood volume, stimulating increased proliferation of blood cells and leading to telomere shortening. Interestingly, weight loss is positively correlated with telomere lengthening and those with shortest telomere length at baseline benefit from the most pronounced rate of telomere lengthening following weight loss. A greater adherence to a Mediterranean diet is also associated with longer telomeres. | [78,79,80,81] |
| Smoking | Telomere length is shorter in smokers and ex-smokers compared to non-smokers and negatively associated with the amount of cigarettes smoked per year. | [78,82] |
| Alcohol consumption | Telomere length is negatively correlated with the number of alcohol units consumed per day and is shorter in alcohol abusers compared to controls. | [83] |
| Premature Aging Disorder | Characteristic Symptoms | Mutations Observed | Effects on Telomere Structure | References |
|---|---|---|---|---|
| Hutchinson-Gilford Progeria Syndrome | Hair greying and loss, decreased joint mobility, loss of subcutaneous fat and atherosclerosis | Point mutation in the LMNA gene encoding prelamin A; a protein involved in nuclear lamina. Mutant LMNA induces DNA damage response at the telomere leading to cell senescence | Shortened telomere length | [91,92,93] |
| Werner Syndrome | Hair greying and loss, skin atrophy, diabetes, osteoporosis, cataracts, arteriosclerosis and neoplasms | Mutation in WRN gene located on the P arm of chromosome 8, which encodes the RecQ DNA helicase involved in DNA replication, recombination and repair. Recruitment of WRN by TERF2 is essential for resolution of the telomeric D-loop and synthesis of the telomeric 3′ overhang | Average telomere length is not reduced. However, loss of telomeres on individual sister chromatids is observed leading to chromosome breakage-fusion events, genome instability and cell senescence. The rate of overall telomere attrition is also increased. | [94,95,96,97,98] |
| Bloom Syndrome | Growth retardation, immunodeficiency, genomic instability cancer and premature menopause | Mutation of BLM; another RecQ helicase associated with TERF2 and involved in DNA replication, recombination and repair | Telomere length is not reduced. However, the rate of telomere shortening is accelerated | [99,100,101,102] |
| Nijmegen Breakage Syndrome | Chromosomal instability and cancers | Mutation of NSB1, which is involved in DNA repair in association with TERF2 | Shortened telomere length | [103,104] |
| Cockayne Syndrome | Neurological degeneration, hearing loss, retinal degeneration and loss of subcutaneous fat | Mutation in one of five genes including CSA, CSB, XPB, XPD and XPG. Mutation in CSB is implicated in the majority of cases. CSB interacts with TERF2 as well as TERF1 to regulate telomere length maintenance | Shortened telomere length | [105,106,107] |
| Dyskeratosis Congenita | Abnormal skin pigmentation, nail dystrophy, bone marrow failure and cancer | One of several mutations involving telomerase (an enzyme involved in telomere length maintenance) or proteins that regulate telomerase. In the X-linked recessive form, DKC1 is mutated, which associates with TERC (the RNA component of telomerase). In the autosomal dominant form, TERC is commonly involved; however TIFN2 is mutated in some cases. In autosomal recessive forms, mutations in TERT (the reverse transcriptase component of telomerase), NOP10 and NHP2 are the cause. NHP2 interacts with NOP10, which in turn associates with DKC1 in order to interact with TERC. | Shortened telomere length. Furthermore, shorter telomeres are associated with more severe clinical phenotypes | [108,109,110,111,112] |
| Ataxia telangiectasia | Neurological deterioration, chromosomal instability and predisposition to cancer | Mutations in ATM, which is located on the q arm of chromosome 11 and is involved in cell cycle progression and DNA repair pathways | Accelerated telomere shortening and chromosome fusion events | [113,114] |
| Down’s Syndrome | Accelerated aging characteristics such as premature skin wrinkling, greying hair, hypogonadism, hypothyroidism, early menopause and declining immune function. In addition overexpression of amyloid precursor protein (APP) on chromosome 21 leads to Alzheimer’s Disease | Trisomy chromosome 21 | Shortened telomere length | [115,116,117] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. https://doi.org/10.3390/cells8010073
Turner KJ, Vasu V, Griffin DK. Telomere Biology and Human Phenotype. Cells. 2019; 8(1):73. https://doi.org/10.3390/cells8010073
Chicago/Turabian StyleTurner, Kara J., Vimal Vasu, and Darren K. Griffin. 2019. "Telomere Biology and Human Phenotype" Cells 8, no. 1: 73. https://doi.org/10.3390/cells8010073
APA StyleTurner, K. J., Vasu, V., & Griffin, D. K. (2019). Telomere Biology and Human Phenotype. Cells, 8(1), 73. https://doi.org/10.3390/cells8010073