Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells
Abstract
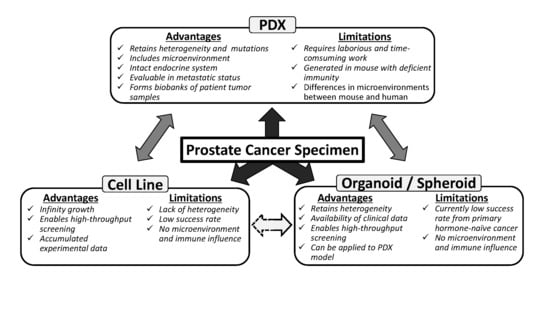
:1. Introduction
2. PCa Cell Lines
2.1. Original PCa Cell Lines Derived from Primary Tumors
2.2. Misclassified Cell Lines
2.3. Original PCa Cell Lines Derived from Metastasis Tumors
2.4. Original PCa Cell Lines Derived from Xenograft Tumors
2.5. PCa Sublines Showing Treatment Resistance
2.5.1. Castration-Resistant PCa Sublines
2.5.2. Antiandrogen-Resistant PCa Sublines
2.5.3. Chemotherapy-Resistant PCa Sublines
3. Patient-Derived Xenografts
3.1. Advancements in the Development of Immunodeficient Mice
3.2. Patient-Derived Xenograft Models
4. Three-Dimensional Cultures of Patient-Derived PCa Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Dehm, S.M.; Schmidt, L.J.; Heemers, H.V.; Vessella, R.L.; Tindall, D.J. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008, 68, 5469–5477. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Q.; Liu, X.; Liu, C.; Liu, R.; Rycaj, K.; Zhang, D.; Liu, B.; Jeter, C.; Calhoun-Davis, T.; et al. Defining a population of stem-like human prostate cancer cells that can generate and propagate castration-resistant prostate cancer. Clin. Cancer Res. 2016, 22, 4505–4516. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Bluemn, E.G.; Coleman, I.M.; Lucas, J.M.; Coleman, R.T.; Hernandez-Lopez, S.; Tharakan, R.; Bianchi-Frias, D.; Dumpit, R.F.; Kaipainen, A.; Corella, A.N.; et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell 2017, 32, 474–489. [Google Scholar] [CrossRef]
- Wang, H.T.; Yao, Y.H.; Li, B.G.; Tang, Y.; Chang, J.W.; Zhang, J. Neuroendocrine prostate cancer (nepc) progressing from conventional prostatic adenocarcinoma: Factors associated with time to development of nepc and survival from NEPC diagnosis-a systematic review and pooled analysis. J. Clin. Oncol. 2014, 32, 3383–3390. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Leong, S.S.; Chu, T.M.; Wajsman, Z.L.; Friedman, M.; Papsidero, L.; Kim, U.; Chai, L.S.; Kakati, S.; Arya, S.K.; et al. The lncap cell line--a new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 1980, 37, 115–132. [Google Scholar]
- Mickey, D.D.; Stone, K.R.; Wunderli, H.; Mickey, G.H.; Vollmer, R.T.; Paulson, D.F. Heterotransplantation of a human prostatic adenocarcinoma cell line in nude mice. Cancer Res. 1977, 37, 4049–4058. [Google Scholar]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (pc-3). Invest. Urol. 1979, 17, 16–23. [Google Scholar] [PubMed]
- Sobel, R.E.; Sadar, M.D. Cell lines used in prostate cancer research: A compendium of old and new lines--part 1. J. Urol. 2005, 173, 342–359. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Misawa, A.; Suzuki, T.; Takagi, K.; Hayashizaki, Y.; Fujimura, T.; Homma, Y.; Takahashi, S.; Urano, T.; Inoue, S. Tet2 repression by androgen hormone regulates global hydroxymethylation status and prostate cancer progression. Nat. Commun. 2015, 6, 8219. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Horie-Inoue, K.; Suzuki, T.; Urano, T.; Ikeda, K.; Fujimura, T.; Takahashi, S.; Homma, Y.; Ouchi, Y.; Inoue, S. Tacc2 is an androgen-responsive cell cycle regulator promoting androgen-mediated and castration-resistant growth of prostate cancer. Mol. Endocrinol. 2012, 26, 748–761. [Google Scholar] [CrossRef]
- Druker, B.J.; Tamura, S.; Buchdunger, E.; Ohno, S.; Segal, G.M.; Fanning, S.; Zimmermann, J.; Lydon, N.B. Effects of a selective inhibitor of the ABL tyrosine kinase on the growth of bcr-abl positive cells. Nat. Med. 1996, 2, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Solit, D.B.; Garraway, L.A.; Pratilas, C.A.; Sawai, A.; Getz, G.; Basso, A.; Ye, Q.; Lobo, J.M.; She, Y.; Osman, I.; et al. Braf mutation predicts sensitivity to mek inhibition. Nature 2006, 439, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Chen, Y. Organoid development in cancer genome discovery. Curr. Opin. Genet. Dev. 2015, 30, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Wilding, J.L.; Bodmer, W.F. Cancer cell lines for drug discovery and development. Cancer Res. 2014, 74, 2377–2384. [Google Scholar] [CrossRef]
- Ledford, H. Us cancer institute to overhaul tumour cell lines. Nature 2016, 530, 391. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Vessella, R.L.; Morrissey, C.; Brown, L.G.; Coleman, I.M.; Higano, C.S.; Mostaghel, E.A.; Zhang, X.; True, L.D.; Lam, H.M.; et al. Lucap prostate cancer patient-derived xenografts reflect the molecular heterogeneity of advanced disease an--d serve as models for evaluating cancer therapeutics. Prostate 2017, 77, 654–671. [Google Scholar] [CrossRef]
- Li, Z.G.; Mathew, P.; Yang, J.; Starbuck, M.W.; Zurita, A.J.; Liu, J.; Sikes, C.; Multani, A.S.; Efstathiou, E.; Lopez, A.; et al. Androgen receptor-negative human prostate cancer cells induce osteogenesis in mice through fgf9-mediated mechanisms. J. Clin. Investig. 2008, 118, 2697–2710. [Google Scholar] [CrossRef] [PubMed]
- van Weerden, W.M.; Bangma, C.; de Wit, R. Human xenograft models as useful tools to assess the potential of novel therapeutics in prostate cancer. Br. J. Cancer 2009, 100, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Establishment of a patient-derived tumor xenograft model and application for precision cancer medicine. Chem. Pharm. Bull. 2018, 66, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Lemos, R.; Powis, G. The promise of patient-derived xenografts: The best laid plans of mice and men. Clin. Cancer Res. 2012, 18, 5160–5162. [Google Scholar] [CrossRef] [PubMed]
- Daniel, V.C.; Marchionni, L.; Hierman, J.S.; Rhodes, J.T.; Devereux, W.L.; Rudin, C.M.; Yung, R.; Parmigiani, G.; Dorsch, M.; Peacock, C.D.; et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009, 69, 3364–3373. [Google Scholar] [CrossRef]
- Navone, N.M.; van Weerden, W.M.; Vessella, R.L.; Williams, E.D.; Wang, Y.; Isaacs, J.T.; Nguyen, H.M.; Culig, Z.; van der Pluijm, G.; Rentsch, C.A.; et al. Movember gap1 pdx project: An international collection of serially transplantable prostate cancer patient-derived xenograft (pdx) models. Prostate 2018, 78, 1262–1282. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Centenera, M.M.; Raj, G.V.; Knudsen, K.E.; Tilley, W.D.; Butler, L.M. Ex vivo culture of human prostate tissue and drug development. Nat. Rev. Urol. 2013, 10, 483–487. [Google Scholar] [CrossRef]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef]
- Masters, J.R. Hela cells 50 years on: The good, the bad and the ugly. Nat. Rev. Cancer 2002, 2, 315–319. [Google Scholar] [CrossRef]
- McDermott, U.; Sharma, S.V.; Dowell, L.; Greninger, P.; Montagut, C.; Lamb, J.; Archibald, H.; Raudales, R.; Tam, A.; Lee, D.; et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc. Natl. Acad. Sci. USA 2007, 104, 19936–19941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culig, Z.; Hoffmann, J.; Erdel, M.; Eder, I.E.; Hobisch, A.; Hittmair, A.; Bartsch, G.; Utermann, G.; Schneider, M.R.; Parczyk, K.; et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br. J. Cancer 1999, 81, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.D. Human urologic cancer cell lines. Invest. Urol. 1980, 17, 359–363. [Google Scholar] [PubMed]
- Williams, R.D.; Bronson, D.L.; Elliott, A.Y.; Gehrke, C.W.; Kuo, K.; Fraley, E.E. Biochemical markers of cultured human prostatic epithelium. J. Urol. 1978, 119, 768–771. [Google Scholar] [CrossRef]
- Billstrom, A.; Lecander, I.; Dagnaes-Hansen, F.; Dahllof, B.; Stenram, U.; Hartley-Asp, B. Differential expression of upa in an aggressive (du 145) and a nonaggressive (1013l) human prostate cancer xenograft. Prostate 1995, 26, 94–104. [Google Scholar] [CrossRef]
- Hartley-Asp, B.; Billstrom, A.; Kruse, E. Identification by c-banding of two human prostate tumour cell lines, 1013l and du 145. Int. J. Cancer 1989, 44, 161–164. [Google Scholar] [CrossRef] [PubMed]
- van Bokhoven, A.; Varella-Garcia, M.; Korch, C.; Johannes, W.U.; Smith, E.E.; Miller, H.L.; Nordeen, S.K.; Miller, G.J.; Lucia, M.S. Molecular characterization of human prostate carcinoma cell lines. Prostate 2003, 57, 205–225. [Google Scholar] [CrossRef]
- Koochekpour, S.; Maresh, G.A.; Katner, A.; Parker-Johnson, K.; Lee, T.J.; Hebert, F.E.; Kao, Y.S.; Skinner, J.; Rayford, W. Establishment and characterization of a primary androgen-responsive african-american prostate cancer cell line, e006aa. Prostate 2004, 60, 141–152. [Google Scholar] [CrossRef]
- Koochekpour, S.; Willard, S.S.; Shourideh, M.; Ali, S.; Liu, C.; Azabdaftari, G.; Saleem, M.; Attwood, K. Establishment and characterization of a highly tumorigenic african american prostate cancer cell line, e006aa-ht. Int. J. Biol. Sci. 2014, 10, 834–845. [Google Scholar] [CrossRef]
- Ilboudo, A.; Chouhan, J.; McNeil, B.K.; Osborne, J.R.; Ogunwobi, O.O. Pvt1 exon 9: A potential biomarker of aggressive prostate cancer? Int. J. Environ. Res. Public Health 2015, 13, 12. [Google Scholar] [CrossRef]
- Theodore, S.; Sharp, S.; Zhou, J.; Turner, T.; Li, H.; Miki, J.; Ji, Y.; Patel, V.; Yates, C.; Rhim, J.S. Establishment and characterization of a pair of non-malignant and malignant tumor derived cell lines from an african american prostate cancer patient. Int. J. Oncol. 2010, 37, 1477–1482. [Google Scholar] [PubMed]
- Woods-Burnham, L.; Basu, A.; Cajigas-Du Ross, C.K.; Love, A.; Yates, C.; De Leon, M.; Roy, S.; Casiano, C.A. The 22rv1 prostate cancer cell line carries mixed genetic ancestry: Implications for prostate cancer health disparities research using pre-clinical models. Prostate 2017, 77, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (du 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef]
- Sherwood, E.R.; Berg, L.A.; Mitchell, N.J.; McNeal, J.E.; Kozlowski, J.M.; Lee, C. Differential cytokeratin expression in normal, hyperplastic and malignant epithelial cells from human prostate. J. Urol. 1990, 143, 167–171. [Google Scholar] [CrossRef]
- Bastide, C.; Bagnis, C.; Mannoni, P.; Hassoun, J.; Bladou, F. A nod scid mouse model to study human prostate cancer. Prostate Cancer Prostatic Dis. 2002, 5, 311–315. [Google Scholar] [CrossRef]
- Lange, T.; Ullrich, S.; Muller, I.; Nentwich, M.F.; Stubke, K.; Feldhaus, S.; Knies, C.; Hellwinkel, O.J.; Vessella, R.L.; Abramjuk, C.; et al. Human prostate cancer in a clinically relevant xenograft mouse model: Identification of beta(1,6)-branched oligosaccharides as a marker of tumor progression. Clin. Cancer Res. 2012, 18, 1364–1373. [Google Scholar] [CrossRef]
- Lange, T.; Kupfernagel, M.; Wicklein, D.; Gebauer, F.; Maar, H.; Brugge, K.; Muller, I.; Simon, R.; Schlomm, T.; Sauter, G.; et al. Aberrant presentation of hpa-reactive carbohydrates implies selectin-independent metastasis formation in human prostate cancer. Clin. Cancer Res. 2014, 20, 1791–1802. [Google Scholar] [CrossRef]
- Ching, K.Z.; Ramsey, E.; Pettigrew, N.; D’Cunha, R.; Jason, M.; Dodd, J.G. Expression of mrna for epidermal growth factor, transforming growth factor-alpha and their receptor in human prostate tissue and cell lines. Mol. Cell Biochem. 1993, 126, 151–158. [Google Scholar] [CrossRef]
- Kozlowski, J.M.; Fidler, I.J.; Campbell, D.; Xu, Z.L.; Kaighn, M.E.; Hart, I.R. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984, 44, 3522–3529. [Google Scholar]
- Wang, M.; Stearns, M.E. Isolation and characterization of pc-3 human prostatic tumor sublines which preferentially metastasize to select organs in s.C.I.D. Mice. Differentiation 1991, 48, 115–125. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. Pc3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, S.; Tamburrino, L.; Nesi, G.; Paglierani, M.; Gelmini, S.; Orlando, C.; Maggi, M.; Forti, G.; Baldi, E. Androgen-responsive and -unresponsive prostate cancer cell lines respond differently to stimuli inducing neuroendocrine differentiation. Int. J. Androl. 2010, 33, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.A.; di Sant’Agnese, P.A.; Huang, L.S.; Xu, H.; Yao, J.L.; Yang, Q.; Liang, S.; Liu, J.; Yu, R.; Cheng, L.; et al. Cd44 expression is a feature of prostatic small cell carcinoma and distinguishes it from its mimickers. Hum. Pathol. 2009, 40, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. Lncap model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Veldscholte, J.; Ris-Stalpers, C.; Kuiper, G.G.; Jenster, G.; Berrevoets, C.; Claassen, E.; van Rooij, H.C.; Trapman, J.; Brinkmann, A.O.; Mulder, E. A mutation in the ligand binding domain of the androgen receptor of human lncap cells affects steroid binding characteristics and response to anti-androgens. Biochem. Biophys. Res. Commun. 1990, 173, 534–540. [Google Scholar] [CrossRef]
- Carroll, A.G.; Voeller, H.J.; Sugars, L.; Gelmann, E.P. P53 oncogene mutations in three human prostate cancer cell lines. Prostate 1993, 23, 123–134. [Google Scholar] [CrossRef]
- Isaacs, W.B.; Carter, B.S.; Ewing, C.M. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991, 51, 4716–4720. [Google Scholar]
- Zhau, H.Y.; Chang, S.M.; Chen, B.Q.; Wang, Y.; Zhang, H.; Kao, C.; Sang, Q.A.; Pathak, S.J.; Chung, L.W. Androgen-repressed phenotype in human prostate cancer. Proc. Natl. Acad. Sci. USA 1996, 93, 15152–15157. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.Y.; Boyle, B.; Krishnan, A.V.; Navone, N.M.; Peehl, D.M.; Feldman, D. Two mutations identified in the androgen receptor of the new human prostate cancer cell line mda pca 2a. J. Urol. 1999, 162, 2192–2199. [Google Scholar] [CrossRef]
- Navone, N.M.; Rodriquez-Vargas, M.C.; Benedict, W.F.; Troncoso, P.; McDonnell, T.J.; Zhou, J.H.; Luthra, R.; Logothetis, C.J. Tabbo: A model reflecting common molecular features of androgen-independent prostate cancer. Clin. Cancer Res. 2000, 6, 1190–1197. [Google Scholar]
- Ellis, W.J.; Vessella, R.L.; Buhler, K.R.; Bladou, F.; True, L.D.; Bigler, S.A.; Curtis, D.; Lange, P.H. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: Lucap 23. Clin. Cancer Res. 1996, 2, 1039–1048. [Google Scholar] [PubMed]
- Sobel, R.E.; Sadar, M.D. Cell lines used in prostate cancer research: A compendium of old and new lines--part 2. J. Urol. 2005, 173, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.A.; Reiter, R.E.; Redula, J.; Moradi, H.; Zhu, X.L.; Brothman, A.R.; Lamb, D.J.; Marcelli, M.; Belldegrun, A.; Witte, O.N.; et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient scid mice. Nat. Med. 1997, 3, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Parris, A.B.; Grossman, G.; Mohler, J.L.; Wang, Z.; Wilson, E.M. Up-regulation of follistatin-like 1 by the androgen receptor and melanoma antigen-a11 in prostate cancer. Prostate 2017, 77, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Masko, E.M.; Alfaqih, M.A.; Solomon, K.R.; Barry, W.T.; Newgard, C.B.; Muehlbauer, M.J.; Valilis, N.A.; Phillips, T.E.; Poulton, S.H.; Freedland, A.R.; et al. Evidence for feedback regulation following cholesterol lowering therapy in a prostate cancer xenograft model. Prostate 2017, 77, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Sramkoski, R.M.; Pretlow, T.G., 2nd; Giaconia, J.M.; Pretlow, T.P.; Schwartz, S.; Sy, M.S.; Marengo, S.R.; Rhim, J.S.; Zhang, D.; Jacobberger, J.W. A new human prostate carcinoma cell line, 22rv1. In Vitro Cell Dev. Biol. Anim. 1999, 35, 403–409. [Google Scholar] [CrossRef]
- Tepper, C.G.; Boucher, D.L.; Ryan, P.E.; Ma, A.H.; Xia, L.; Lee, L.F.; Pretlow, T.G.; Kung, H.J. Characterization of a novel androgen receptor mutation in a relapsed cwr22 prostate cancer xenograft and cell line. Cancer Res. 2002, 62, 6606–6614. [Google Scholar]
- Li, Y.; Alsagabi, M.; Fan, D.; Bova, G.S.; Tewfik, A.H.; Dehm, S.M. Intragenic rearrangement and altered rna splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res. 2011, 71, 2108–2117. [Google Scholar] [CrossRef]
- Rubin, M.A.; Putzi, M.; Mucci, N.; Smith, D.C.; Wojno, K.; Korenchuk, S.; Pienta, K.J. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin. Cancer Res. 2000, 6, 1038–1045. [Google Scholar]
- Korenchuk, S.; Lehr, J.E.; MClean, L.; Lee, Y.G.; Whitney, S.; Vessella, R.; Lin, D.L.; Pienta, K.J. Vcap, a cell-based model system of human prostate cancer. In Vivo 2001, 15, 163–168. [Google Scholar]
- Yoshida, T.; Kinoshita, H.; Segawa, T.; Nakamura, E.; Inoue, T.; Shimizu, Y.; Kamoto, T.; Ogawa, O. Antiandrogen bicalutamide promotes tumor growth in a novel androgen-dependent prostate cancer xenograft model derived from a bicalutamide-treated patient. Cancer Res. 2005, 65, 9611–9616. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Shimizu, Y.; Yoshida, T.; Maeno, A.; Kamba, T.; Inoue, T.; Nakamura, E.; Kamoto, T.; Ogawa, O. Antiandrogen withdrawal syndrome and alternative antiandrogen therapy associated with the w741c mutant androgen receptor in a novel prostate cancer xenograft. Prostate 2010, 70, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Lejeune, P.; Kohr, S.; Neuhaus, R.; Faus, H.; Gelato, K.A.; Busemann, M.; Cleve, A.; Lucking, U.; von Nussbaum, F.; et al. Bay 1024767 blocks androgen receptor mutants found in castration-resistant prostate cancer patients. Oncotarget 2016, 7, 6015–6028. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.; Jones, D.; Wade, M.; Grey, J.; Nakjang, S.; Guo, W.; Cork, D.; Davies, B.R.; Wedge, S.R.; Robson, C.N.; et al. Development and exploitation of a novel mutant androgen receptor modelling strategy to identify new targets for advanced prostate cancer therapy. Oncotarget 2015, 6, 26029–26040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, R.B.; van Weerden, W.M.; Erkens-Schulze, S.; de Ridder, C.M.; Bangma, C.H.; Trapman, J.; Jenster, G. The human pc346 xenograft and cell line panel: A model system for prostate cancer progression. Eur. Urol. 2006, 49, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, J.; Takashima, M.; Koh, E.; Namiki, M. Androgen-independent growth in lncap cell lines and steroid uridine diphosphate-glucuronosyltransferase expression. Asian J. Androl. 2003, 5, 9–13. [Google Scholar] [PubMed]
- Iwasa, Y.; Mizokami, A.; Miwa, S.; Koshida, K.; Namiki, M. Establishment and characterization of androgen-independent human prostate cancer cell lines, ln-rec4 and lncap-sf, from lncap. Int. J. Urol. 2007, 14, 233–239. [Google Scholar] [CrossRef]
- Takayama, K.; Horie-Inoue, K.; Katayama, S.; Suzuki, T.; Tsutsumi, S.; Ikeda, K.; Urano, T.; Fujimura, T.; Takagi, K.; Takahashi, S.; et al. Androgen-responsive long noncoding rna ctbp1-as promotes prostate cancer. EMBO J. 2013, 32, 1665–1680. [Google Scholar] [CrossRef]
- Misawa, A.; Takayama, K.; Urano, T.; Inoue, S. Androgen-induced long noncoding rna (lncrna) socs2-as1 promotes cell growth and inhibits apoptosis in prostate cancer cells. J. Biol. Chem. 2016, 291, 17861–17880. [Google Scholar] [CrossRef]
- Wu, H.C.; Hsieh, J.T.; Gleave, M.E.; Brown, N.M.; Pathak, S.; Chung, L.W. Derivation of androgen-independent human lncap prostatic cancer cell sublines: Role of bone stromal cells. Int. J. Cancer 1994, 57, 406–412. [Google Scholar] [CrossRef]
- Thalmann, G.N.; Anezinis, P.E.; Chang, S.M.; Zhau, H.E.; Kim, E.E.; Hopwood, V.L.; Pathak, S.; von Eschenbach, A.C.; Chung, L.W. Androgen-independent cancer progression and bone metastasis in the lncap model of human prostate cancer. Cancer Res. 1994, 54, 2577–2581. [Google Scholar] [PubMed]
- Marques, R.B.; Dits, N.F.; Erkens-Schulze, S.; van Ijcken, W.F.; van Weerden, W.M.; Jenster, G. Modulation of androgen receptor signaling in hormonal therapy-resistant prostate cancer cell lines. PLoS ONE 2011, 6, e23144. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Armstrong, C.M.; Lou, W.; Lombard, A.P.; Cucchiara, V.; Gu, X.; Yang, J.C.; Nadiminty, N.; Pan, C.X.; Evans, C.P.; et al. Niclosamide and bicalutamide combination treatment overcomes enzalutamide- and bicalutamide-resistant prostate cancer. Mol. Cancer Ther. 2017, 16, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Kuruma, H.; Matsumoto, H.; Shiota, M.; Bishop, J.; Lamoureux, F.; Thomas, C.; Briere, D.; Los, G.; Gleave, M.; Fanjul, A.; et al. A novel antiandrogen, compound 30, suppresses castration-resistant and mdv3100-resistant prostate cancer growth in vitro and in vivo. Mol. Cancer Ther. 2013, 12, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, H.; Lallous, N.; Ozistanbullu, D.; Beraldi, E.; Paul, N.; Dalal, K.; Fazli, L.; Haferkamp, A.; Lejeune, P.; Cherkasov, A.; et al. Moving towards precision urologic oncology: Targeting enzalutamide-resistant prostate cancer and mutated forms of the androgen receptor using the novel inhibitor darolutamide (odm-201). Eur. Urol. 2018, 73, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Thaper, D.; Vahid, S.; Davies, A.; Ketola, K.; Kuruma, H.; Jama, R.; Nip, K.M.; Angeles, A.; Johnson, F.; et al. The master neural transcription factor brn2 is an androgen receptor-suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov. 2017, 7, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Mizokami, A.; Mamiya, K.; Li, Y.Q.; Zhang, J.; Keller, E.T.; Namiki, M. The establishment of two paclitaxel-resistant prostate cancer cell lines and the mechanisms of paclitaxel resistance with two cell lines. Prostate 2007, 67, 955–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Mizokami, A.; Izumi, K.; Narimoto, K.; Shima, T.; Zhang, J.; Dai, J.; Keller, E.T.; Namiki, M. Cten/tensin 4 expression induces sensitivity to paclitaxel in prostate cancer. Prostate 2010, 70, 48–60. [Google Scholar] [CrossRef]
- Souchek, J.J.; Davis, A.L.; Hill, T.K.; Holmes, M.B.; Qi, B.; Singh, P.K.; Kridel, S.J.; Mohs, A.M. Combination treatment with orlistat-containing nanoparticles and taxanes is synergistic and enhances microtubule stability in taxane-resistant prostate cancer cells. Mol. Cancer Ther. 2017, 16, 1819–1830. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Wang, L.; Mizokami, A.; Keller, E.T.; Zhang, J.; Fu, J. Skp2 is associated with paclitaxel resistance in prostate cancer cells. Oncol. Rep. 2016, 36, 559–566. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, M.; Zeng, Y.; Li, Y.; Zhang, C.; Getzenberg, R.H.; Song, Y. Downregulation of cytokeratin 18 is associated with paclitaxelresistance and tumor aggressiveness in prostate cancer. Int. J. Oncol. 2016, 48, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, G.; Getzenberg, R.H.; Veltri, R.W. The upregulation of pi3k/akt and map kinase pathways is associated with resistance of microtubule-targeting drugs in prostate cancer. J. Cell Biochem. 2015, 116, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Yin, B.; Christudass, C.S.; Terada, N.; Rajagopalan, K.; Fabry, B.; Lee, D.Y.; Shiraishi, T.; Getzenberg, R.H.; Veltri, R.W.; et al. Acquisition of paclitaxel resistance is associated with a more aggressive and invasive phenotype in prostate cancer. J. Cell Biochem. 2013, 114, 1286–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Chitkara, D.; Mehrazin, R.; Behrman, S.W.; Wake, R.W.; Mahato, R.I. Chemoresistance in prostate cancer cells is regulated by mirnas and hedgehog pathway. PLoS ONE 2012, 7, e40021. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Danquah, M.; Singh, S.; Wu, H.; Mahato, R.I. Paclitaxel- and lapatinib-loaded lipopolymer micelles overcome multidrug resistance in prostate cancer. Drug Deliv. Transl. Res. 2011, 1, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, Y.; Mooney, S.M.; Yin, B.; Mizokami, A.; Namiki, M.; Getzenberg, R.H. Resistance to paclitaxel increases the sensitivity to other microenvironmental stresses in prostate cancer cells. J. Cell Biochem. 2011, 112, 2125–2137. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Kojima, K.; Ohhashi, R.; Hamada, N.; Nozawa, Y.; Kitamoto, A.; Sato, A.; Kondo, S.; Kojima, T.; Deguchi, T.; et al. Mir-148a attenuates paclitaxel resistance of hormone-refractory, drug-resistant prostate cancer pc3 cells by regulating msk1 expression. J. Biol. Chem. 2010, 285, 19076–19084. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Fujita, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. Mir-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer pc3 cells through direct and indirect mechanisms. Prostate 2010, 70, 1501–1512. [Google Scholar] [CrossRef]
- Kato, T.; Fujita, Y.; Nakane, K.; Kojima, T.; Nozawa, Y.; Deguchi, T.; Ito, M. Ets1 promotes chemoresistance and invasion of paclitaxel-resistant, hormone-refractory pc3 prostate cancer cells by up-regulating mdr1 and mmp9 expression. Biochem. Biophys. Res. Commun. 2012, 417, 966–971. [Google Scholar] [CrossRef]
- Kato, T.; Fujita, Y.; Nakane, K.; Mizutani, K.; Terazawa, R.; Ehara, H.; Kanimoto, Y.; Kojima, T.; Nozawa, Y.; Deguchi, T.; et al. Ccr1/ccl5 interaction promotes invasion of taxane-resistant pc3 prostate cancer cells by increasing secretion of mmps 2/9 and by activating erk and rac signaling. Cytokine 2013, 64, 251–257. [Google Scholar] [CrossRef]
- Byun, W.S.; Jin, M.; Yu, J.; Kim, W.K.; Song, J.; Chung, H.J.; Jeong, L.S.; Lee, S.K. A novel selenonucleoside suppresses tumor growth by targeting skp2 degradation in paclitaxel-resistant prostate cancer. Biochem. Pharmacol. 2018, 158, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sowery, R.D.; Hadaschik, B.A.; So, A.I.; Zoubeidi, A.; Fazli, L.; Hurtado-Coll, A.; Gleave, M.E. Clusterin knockdown using the antisense oligonucleotide ogx-011 re-sensitizes docetaxel-refractory prostate cancer pc-3 cells to chemotherapy. BJU Int. 2008, 102, 389–397. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.J.; Prencipe, M.; Dowling, C.; Fan, Y.; Mulrane, L.; Gallagher, W.M.; O’Connor, D.; O’Connor, R.; Devery, A.; Corcoran, C.; et al. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol. Cancer 2011, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, K.; O’Neill, A.; Prencipe, M.; Bugler, J.; Murphy, L.; Fabre, A.; Puhr, M.; Culig, Z.; Murphy, K.; Watson, R.W. The role of epithelial-mesenchymal transition drivers zeb1 and zeb2 in mediating docetaxel-resistant prostate cancer. Mol. Oncol. 2017, 11, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Marin-Aguilera, M.; Codony-Servat, J.; Kalko, S.G.; Fernandez, P.L.; Bermudo, R.; Buxo, E.; Ribal, M.J.; Gascon, P.; Mellado, B. Identification of docetaxel resistance genes in castration-resistant prostate cancer. Mol. Cancer Ther. 2012, 11, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Hongo, H.; Kosaka, T.; Oya, M. Analysis of cabazitaxel-resistant mechanism in human castration-resistant prostate cancer. Cancer Sci. 2018, 109, 2937–2945. [Google Scholar] [CrossRef]
- Machioka, K.; Izumi, K.; Kadono, Y.; Iwamoto, H.; Naito, R.; Makino, T.; Kadomoto, S.; Natsagdorj, A.; Keller, E.T.; Zhang, J.; et al. Establishment and characterization of two cabazitaxel-resistant prostate cancer cell lines. Oncotarget 2018, 9, 16185–16196. [Google Scholar] [CrossRef]
- van Bokhoven, A.; Varella-Garcia, M.; Korch, C.; Hessels, D.; Miller, G.J. Widely used prostate carcinoma cell lines share common origins. Prostate 2001, 47, 36–51. [Google Scholar] [CrossRef]
- Varella-Garcia, M.; Boomer, T.; Miller, G.J. Karyotypic similarity identified by multiplex-fish relates four prostate adenocarcinoma cell lines: Pc-3, ppc-1, alva-31, and alva-41. Genes Chromosomes Cancer 2001, 31, 303–315. [Google Scholar] [CrossRef]
- MacLeod, R.A.; Dirks, W.G.; Matsuo, Y.; Kaufmann, M.; Milch, H.; Drexler, H.G. Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int. J. Cancer 1999, 83, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Loop, S.M.; Rozanski, T.A.; Ostenson, R.C. Human primary prostate tumor cell line, alva-31: A new model for studying the hormonal regulation of prostate tumor cell growth. Prostate 1993, 22, 93–108. [Google Scholar] [CrossRef]
- Brothman, A.R.; Lesho, L.J.; Somers, K.D.; Wright, G.L., Jr.; Merchant, D.J. Phenotypic and cytogenetic characterization of a cell line derived from primary prostatic carcinoma. Int. J. Cancer 1989, 44, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.R. Chromosome identity of human prostate cancer cell lines, pc-3 and ppc-1. Cytogenet. Cell Genet. 1993, 62, 183–184. [Google Scholar] [CrossRef] [PubMed]
- van Helden, P.D.; Wiid, I.J.; Hoal-van Helden, E.G.; Bey, E.; Cohen, R. Detection by DNA fingerprinting of somatic changes during the establishment of a new prostate cell line. Br. J. Cancer 1994, 70, 195–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, P.; Dahiya, R. Establishment and characterization of a human primary prostatic adenocarcinoma cell line (nd-1). J. Urol. 1992, 148, 1600–1604. [Google Scholar] [CrossRef]
- Muraki, J.; Addonizio, J.C.; Choudhury, M.S.; Fischer, J.; Eshghi, M.; Davidian, M.M.; Shapiro, L.R.; Wilmot, P.L.; Nagamatsu, G.R.; Chiao, J.W. Establishment of new human prostatic cancer cell line (jca-1). Urology 1990, 36, 79–84. [Google Scholar] [CrossRef]
- van Bokhoven, A.; Varella-Garcia, M.; Korch, C.; Miller, G.J. Tsu-pr1 and jca-1 cells are derivatives of t24 bladder carcinoma cells and are not of prostatic origin. Cancer Res. 2001, 61, 6340–6344. [Google Scholar]
- Claas, F.H.; van Steenbrugge, G.J. Expression of hla-like structures on a permanent human tumor line pc-93. Tissue Antigens 1983, 21, 227–232. [Google Scholar] [CrossRef]
- Schmelz, M.; Cress, A.E.; Barrera, J.; McDaniel, K.M.; Davis, T.L.; Fuchs, L.; Dalkin, B.L.; Nagle, R.B. Peaz-1: A new human prostate neoplastic epithelial cell line. Prostate 2001, 48, 79–92. [Google Scholar] [CrossRef]
- Nomura, T.; Yamasaki, M.; Nomura, Y.; Mimata, H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol. Rep. 2005, 14, 993–997. [Google Scholar] [CrossRef]
- van Weerden, W.M.; de Ridder, C.M.; Verdaasdonk, C.L.; Romijn, J.C.; van der Kwast, T.H.; Schroder, F.H.; van Steenbrugge, G.J. Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am. J. Pathol. 1996, 149, 1055–1062. [Google Scholar] [PubMed]
- Fridman, R.; Giaccone, G.; Kanemoto, T.; Martin, G.R.; Gazdar, A.F.; Mulshine, J.L. Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc. Natl. Acad. Sci. USA 1990, 87, 6698–6702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Revelo, M.P.; Sudilovsky, D.; Cao, M.; Chen, W.G.; Goetz, L.; Xue, H.; Sadar, M.; Shappell, S.B.; Cunha, G.R.; et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate 2005, 64, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Toivanen, R.; Berman, D.M.; Wang, H.; Pedersen, J.; Frydenberg, M.; Meeker, A.K.; Ellem, S.J.; Risbridger, G.P.; Taylor, R.A. Brief report: A bioassay to identify primary human prostate cancer repopulating cells. Stem Cells 2011, 29, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, W.; Schroeder, F.H.; Reimann, J.F.; Joebsis, A.C.; Hermanek, P. Human prostatic adenocarcinoma: Some characteristics of a serially transplantable line in nude mice (pc 82). Prostate 1980, 1, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, W.; Wagner, M.; Riemann, J.F.; Hermanek, P.; Williams, E.; Walther, R.; Schrueffer, R. Prostatic adenocarcinoma pc ew, a new human tumor line transplantable in nude mice. Prostate 1984, 5, 445–452. [Google Scholar] [CrossRef] [PubMed]
- van Weerden, W.M.; Romijn, J.C. Use of nude mouse xenograft models in prostate cancer research. Prostate 2000, 43, 263–271. [Google Scholar] [CrossRef]
- Kiefer, J.A.; Vessella, R.L.; Quinn, J.E.; Odman, A.M.; Zhang, J.; Keller, E.T.; Kostenuik, P.J.; Dunstan, C.R.; Corey, E. The effect of osteoprotegerin administration on the intra-tibial growth of the osteoblastic lucap 23.1 prostate cancer xenograft. Clin. Exp. Metastasis 2004, 21, 381–387. [Google Scholar] [CrossRef]
- Corey, E.; Quinn, J.E.; Buhler, K.R.; Nelson, P.S.; Macoska, J.A.; True, L.D.; Vessella, R.L. Lucap 35: A new model of prostate cancer progression to androgen independence. Prostate 2003, 55, 239–246. [Google Scholar] [CrossRef]
- Corey, E.; Quinn, J.E.; Bladou, F.; Brown, L.G.; Roudier, M.P.; Brown, J.M.; Buhler, K.R.; Vessella, R.L. Establishment and characterization of osseous prostate cancer models: Intra-tibial injection of human prostate cancer cells. Prostate 2002, 52, 20–33. [Google Scholar] [CrossRef]
- Winters, B.; Brown, L.; Coleman, I.; Nguyen, H.; Minas, T.Z.; Kollath, L.; Vasioukhin, V.; Nelson, P.; Corey, E.; Uren, A.; et al. Inhibition of erg activity in patient-derived prostate cancer xenografts by yk-4-279. Anticancer Res. 2017, 37, 3385–3396. [Google Scholar] [PubMed]
- Lam, H.M.; McMullin, R.; Nguyen, H.M.; Coleman, I.; Gormley, M.; Gulati, R.; Brown, L.G.; Holt, S.K.; Li, W.; Ricci, D.S.; et al. Characterization of an abiraterone ultraresponsive phenotype in castration-resistant prostate cancer patient-derived xenografts. Clin. Cancer Res. 2017, 23, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Stangelberger, A.; Schally, A.V.; Letsch, M.; Szepeshazi, K.; Nagy, A.; Halmos, G.; Kanashiro, C.A.; Corey, E.; Vessella, R. Targeted chemotherapy with cytotoxic bombesin analogue an-215 inhibits growth of experimental human prostate cancers. Int. J. Cancer 2006, 118, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lucas, J.M.; Lam, H.M.; Dumpit, R.; Corey, E.; Chery, L.; et al. Srrm4 expression and the loss of rest activity may promote the emergence of the neuroendocrine phenotype in castration-resistant prostate cancer. Clin. Cancer Res. 2015, 21, 4698–4708. [Google Scholar] [CrossRef] [PubMed]
- True, L.D.; Buhler, K.; Quinn, J.; Williams, E.; Nelson, P.S.; Clegg, N.; Macoska, J.A.; Norwood, T.; Liu, A.; Ellis, W.; et al. A neuroendocrine/small cell prostate carcinoma xenograft-lucap 49. Am. J. Pathol. 2002, 161, 705–715. [Google Scholar] [CrossRef]
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 inhibits osseous prostate cancer growth by dual targeting of cancer cells and bone microenvironment in mouse models. Clin. Cancer Res. 2017, 23, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Garcia, J.; Chan, E.; de la Cruz, C.; Segal, E.; Merchant, M.; Kharbanda, S.; Raisner, R.; Haverty, P.M.; Modrusan, Z.; et al. Therapeutic targeting of the CBP/p300 bromodomain blocks the growth of castration-resistant prostate cancer. Cancer Res. 2017, 77, 5564–5575. [Google Scholar] [CrossRef] [PubMed]
- Lasko, L.M.; Jakob, C.G.; Edalji, R.P.; Qiu, W.; Montgomery, D.; Digiammarino, E.L.; Hansen, T.M.; Risi, R.M.; Frey, R.; Manaves, V.; et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 2017, 550, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hwang, T.H.; Oseth, L.A.; Hauge, A.; Vessella, R.L.; Schmechel, S.C.; Hirsch, B.; Beckman, K.B.; Silverstein, K.A.; Dehm, S.M. Ar intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene 2012, 31, 4759–4767. [Google Scholar] [CrossRef] [PubMed]
- Thadani-Mulero, M.; Portella, L.; Sun, S.; Sung, M.; Matov, A.; Vessella, R.L.; Corey, E.; Nanus, D.M.; Plymate, S.R.; Giannakakou, P. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. Cancer Res. 2014, 74, 2270–2282. [Google Scholar] [CrossRef]
- Craft, N.; Chhor, C.; Tran, C.; Belldegrun, A.; DeKernion, J.; Witte, O.N.; Said, J.; Reiter, R.E.; Sawyers, C.L. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 1999, 59, 5030–5036. [Google Scholar] [PubMed]
- McCulloch, D.R.; Opeskin, K.; Thompson, E.W.; Williams, E.D. Bm18: A novel androgen-dependent human prostate cancer xenograft model derived from a bone metastasis. Prostate 2005, 65, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bakht, M.K.; Derecichei, I.; Li, Y.; Ferraiuolo, R.M.; Dunning, M.J.; Oh, S.W.; Hussein, A.; Youn, H.; Stringer, K.F.; Jeong, C.W.; et al. Neuroendocrine differentiation of prostate cancer leads to psma suppression. Endocr. Relat. Cancer 2018, 26, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Yang, K.; Wang, Y.Z.; Lin, D. Tmem45b is a novel predictive biomarker for prostate cancer progression and metastasis. Neoplasma 2018, 65, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ci, X.; Xue, H.; Wu, R.; Dong, X.; Choi, S.Y.C.; He, H.; Wang, Y.; Zhang, F.; Qu, S.; et al. Patient-derived hormone-naive prostate cancer xenograft models reveal growth factor receptor bound protein 10 as an androgen receptor-repressed gene driving the development of castration-resistant prostate cancer. Eur. Urol. 2018, 73, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Ettinger, S.L.; Qu, S.; Xue, H.; Nabavi, N.; Choi, S.Y.C.; Bell, R.H.; Mo, F.; Haegert, A.M.; Gout, P.W.; et al. Metabolic heterogeneity signature of primary treatment-naive prostate cancer. Oncotarget 2017, 8, 25928–25941. [Google Scholar] [PubMed]
- Lin, D.; Wyatt, A.W.; Xue, H.; Wang, Y.; Dong, X.; Haegert, A.; Wu, R.; Brahmbhatt, S.; Mo, F.; Jong, L.; et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 2014, 74, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Lin, D.; Takhar, M.; Ramnarine, V.R.; Dong, X.; Bell, R.H.; Volik, S.V.; Wang, K.; Xue, H.; Wang, Y.; et al. Stromal gene expression is predictive for metastatic primary prostate cancer. Eur. Urol. 2018, 73, 524–532. [Google Scholar] [CrossRef]
- Qu, S.; Ci, X.; Xue, H.; Dong, X.; Hao, J.; Lin, D.; Clermont, P.L.; Wu, R.; Collins, C.C.; Gout, P.W.; et al. Treatment with docetaxel in combination with aneustat leads to potent inhibition of metastasis in a patient-derived xenograft model of advanced prostate cancer. Br. J. Cancer 2018, 118, 802–812. [Google Scholar] [CrossRef]
- Ci, X.; Hao, J.; Dong, X.; Choi, S.Y.; Xue, H.; Wu, R.; Qu, S.; Gout, P.W.; Zhang, F.; Haegert, A.M.; et al. Heterochromatin protein 1alpha mediates development and aggressiveness of neuroendocrine prostate cancer. Cancer Res. 2018, 78, 2691–2704. [Google Scholar] [CrossRef]
- Terada, N.; Shimizu, Y.; Kamba, T.; Inoue, T.; Maeno, A.; Kobayashi, T.; Nakamura, E.; Kamoto, T.; Kanaji, T.; Maruyama, T.; et al. Identification of ep4 as a potential target for the treatment of castration-resistant prostate cancer using a novel xenograft model. Cancer Res. 2010, 70, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chang, W.; Yang, G.; Ren, C.; Park, S.; Karantanos, T.; Karanika, S.; Wang, J.; Yin, J.; Shah, P.K.; et al. Targeting poly(adp-ribose) polymerase and the c-myb-regulated DNA damage response pathway in castration-resistant prostate cancer. Sci. Signal 2014, 7, ra47. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, A.; Tzelepi, V.; Araujo, J.C.; Guo, C.C.; Liang, S.; Troncoso, P.; Logothetis, C.J.; Navone, N.M.; Maity, S.N. Neuroendocrine prostate cancer xenografts with large-cell and small-cell features derived from a single patient’s tumor: Morphological, immunohistochemical, and gene expression profiles. Prostate 2011, 71, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Tzelepi, V.; Zhang, J.; Lu, J.F.; Kleb, B.; Wu, G.; Wan, X.; Hoang, A.; Efstathiou, E.; Sircar, K.; Navone, N.M.; et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clin. Cancer Res. 2012, 18, 666–677. [Google Scholar] [CrossRef]
- Kleb, B.; Estecio, M.R.; Zhang, J.; Tzelepi, V.; Chung, W.; Jelinek, J.; Navone, N.M.; Tahir, S.; Marquez, V.E.; Issa, J.P.; et al. Differentially methylated genes and androgen receptor re-expression in small cell prostate carcinomas. Epigenetics 2016, 11, 184–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.C.; Gajdosik, M.S.; Josic, D.; Clifton, J.G.; Logothetis, C.; Yu-Lee, L.Y.; Gallick, G.E.; Maity, S.N.; Lin, S.H. Secretome analysis of an osteogenic prostate tumor identifies complex signaling networks mediating cross-talk of cancer and stromal cells within the tumor microenvironment. Mol. Cell Proteom. 2015, 14, 471–483. [Google Scholar] [CrossRef]
- Fong, E.L.; Martinez, M.; Yang, J.; Mikos, A.G.; Navone, N.M.; Harrington, D.A.; Farach-Carson, M.C. Hydrogel-based 3d model of patient-derived prostate xenograft tumors suitable for drug screening. Mol. Pharm. 2014, 11, 2040–2050. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Taylor, R.A.; Toivanen, R.; Pedersen, J.; Norden, S.; Pook, D.W.; Frydenberg, M.; Papargiris, M.M.; Niranjan, B.; Richards, M.G.; et al. A preclinical xenograft model of prostate cancer using human tumors. Nat. Protoc. 2013, 8, 836–848. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Pook, D.W.; Wang, H.; Porter, L.H.; Frydenberg, M.; Kourambas, J.; Appu, S.; Poole, C.; Beardsley, E.K.; Ryan, A.; et al. Establishment of primary patient-derived xenografts of palliative turp specimens to study castrate-resistant prostate cancer. Prostate 2015, 75, 1475–1483. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Obinata, D.; Sandhu, S.; Selth, L.A.; Wong, S.Q.; Porter, L.H.; Lister, N.; Pook, D.; Pezaro, C.J.; Goode, D.L.; et al. Patient-derived models of abiraterone- and enzalutamide-resistant prostate cancer reveal sensitivity to ribosome-directed therapy. Eur. Urol. 2018, 74, 562–572. [Google Scholar] [CrossRef]
- Porter, L.H.; Hashimoto, K.; Lawrence, M.G.; Pezaro, C.; Clouston, D.; Wang, H.; Papargiris, M.; Thorne, H.; Li, J.; Ryan, A.; et al. Intraductal carcinoma of the prostate can evade androgen deprivation, with emergence of castrate-tolerant cells. BJU Int. 2018, 121, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.P. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 1966, 8, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Kikutani, H.; Makino, S. The murine autoimmune diabetes model: Nod and related strains. Adv. Immunol. 1992, 51, 285–322. [Google Scholar] [PubMed]
- Iwamoto, C.; Takenaka, K.; Urata, S.; Yamauchi, T.; Shima, T.; Kuriyama, T.; Daitoku, S.; Saito, Y.; Miyamoto, T.; Iwasaki, H.; et al. The balb/c-specific polymorphic sirpa enhances its affinity for human cd47, inhibiting phagocytosis against human cells to promote xenogeneic engraftment. Exp. Hematol. 2014, 42, 163–171.e161. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, A.; Vormoor, J.; Hanenberg, H.; Wang, J.C.; Bhatia, M.; Lapidot, T.; Moritz, T.; Murdoch, B.; Xiao, X.L.; Kato, I.; et al. Identification of primitive human hematopoietic cells capable of repopulating nod/scid mouse bone marrow: Implications for gene therapy. Nat. Med. 1996, 2, 1329–1337. [Google Scholar] [CrossRef]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. Nod/scid/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human lymphoid and myeloid cell development in nod/ltsz-scid il2r gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005, 174, 6477–6489. [Google Scholar] [CrossRef]
- Krupski, T.; Harding, M.A.; Herce, M.E.; Gulding, K.M.; Stoler, M.H.; Theodorescu, D. The role of vascular endothelial growth factor in the tissue specific in vivo growth of prostate cancer cells. Growth Factors 2001, 18, 287–302. [Google Scholar] [CrossRef]
- Shultz, L.D.; Brehm, M.A.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized mice for immune system investigation: Progress, promise and challenges. Nat. Rev. Immunol. 2012, 12, 786–798. [Google Scholar] [CrossRef]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Huch, M.; van Boxtel, R.; Karthaus, W.; Wedge, D.C.; Tamuri, A.U.; Martincorena, I.; Petljak, M.; Alexandrov, L.B.; Gundem, G.; et al. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature 2014, 513, 422–425. [Google Scholar] [CrossRef] [Green Version]
- Boj, S.F.; Hwang, C.I.; Baker, L.A.; Chio, I.I.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Ohata, H.; Ishiguro, T.; Aihara, Y.; Sato, A.; Sakai, H.; Sekine, S.; Taniguchi, H.; Akasu, T.; Fujita, S.; Nakagama, H.; et al. Induction of the stem-like cell regulator cd44 by rho kinase inhibition contributes to the maintenance of colon cancer-initiating cells. Cancer Res. 2012, 72, 5101–5110. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Sato, A.; Ohata, H.; Ikarashi, Y.; Takahashi, R.U.; Ochiya, T.; Yoshida, M.; Tsuda, H.; Onda, T.; Kato, T.; et al. Establishment and characterization of an in vitro model of ovarian cancer stem-like cells with an enhanced proliferative capacity. Cancer Res. 2016, 76, 150–160. [Google Scholar] [CrossRef]
- Linxweiler, J.; Hammer, M.; Muhs, S.; Kohn, M.; Pryalukhin, A.; Veith, C.; Bohle, R.M.; Stockle, M.; Junker, K.; Saar, M. Patient-derived, three-dimensional spheroid cultures provide a versatile translational model for the study of organ-confined prostate cancer. J. Cancer Res. Clin. Oncol. 2018. [Google Scholar] [CrossRef]
- Drost, J.; Karthaus, W.R.; Gao, D.; Driehuis, E.; Sawyers, C.L.; Chen, Y.; Clevers, H. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 2016, 11, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Puca, L.; Bareja, R.; Prandi, D.; Shaw, R.; Benelli, M.; Karthaus, W.R.; Hess, J.; Sigouros, M.; Donoghue, A.; Kossai, M.; et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 2018, 9, 2404. [Google Scholar] [CrossRef]

| Name | Pathology | Origin | Race | Pretreatment | AR | PSA | First Report Year | References |
|---|---|---|---|---|---|---|---|---|
| 1013L | Adeno | primary | unknown | none | - | - | 1980 | [33,34,35,36,37] |
| E006AA | Adeno | primary | AA | none | + | ± | 2004 | [38,39,40] |
| RC-77T/E | Adeno | primary | AA | none | + | + | 2010 | [41,42] |
| DU-145 | Adeno | metastasis | Caucasian | none | - | - | 1975 | [10,43,44,45,46,47] |
| PC-3 | Adeno | metastasis | Caucasian | none | - | - | 1979 | [11,48,49,50,51,52,53] |
| LNCaP | Adeno | metastasis | Caucasian | none | + | + | 1980 | [9,12,54,55,56,57] |
| ARCaP | Adeno | metastasis | Caucasian | none | ± | ± | 1996 | [58] |
| MDA PCA 2a/b | Adeno | metastasis | AA | ADT | ± | + | 1997 | [59,60] |
| LuCap 23 | Adeno | xenograft tumor from metastasis | Caucasian | ADT, chemotherapy | + | + | 1996 | [46,61,62] |
| LAPC-4 | Adeno | xenograft tumor from metastasis | Caucasian | ADT | + | + | 1997 | [63,64,65] |
| 22Rv1 | Adeno | xenograft tumor from primary tumor | Caucasian | none | + | + | 1999 | [2,66,67,68] |
| VCaP | Adeno | xenograft tumor from metastasis | Caucasian | unknown | + | + | 2001 | [69,70] |
| KUCaP | Adeno | xenograft tumor from metastasis | Asian | ADT | + | + | 2005 | [71,72,73,74] |
| PC346 | Adeno | xenograft tumor from primary tumor | Caucasian | ADT | + | + | 2006 | [75] |
| Name | Character | Parent Cells | Treatment | Method | First Report Year | References |
|---|---|---|---|---|---|---|
| LNCaP-abl | Cas R | LNCaP | castration | culture in androgen depleted medium | 1999 | [32] |
| LNCaP-SF | Cas R | LNCaP | castration | culture in androgen depleted medium | 2003 | [76,77] |
| LNCaP-LTAD | Cas R | LNCaP | castration | culture in androgen depleted medium | 2012 | [14,78,79] |
| C4-2 | Cas R | LNCaP | castration | derived from xenograft tumor in castrated mouse | 1994 | [80,81] |
| PC346Flu1/2 | AA R | PC346 | castration and flutamide | culture in androgen depleted medium with flutamide | 2011 | [82] |
| LNCaP-BicR (Takayama) | AA R | LNCaP | bicalutamide | culture with flutamide | 2015 | [13] |
| LNCaP-BicR (Liu) | AA R | LNCaP | bicalutamide | culture with flutamide | 2017 | [83] |
| MR49F | AA R | LNCaP | enzalutamide | derived from xenograft tumor treated with enzalutamide | 2013 | [84,85] |
| ENZR cell line series | AA R, NEPC | LNCaP | enzalutamide | derived from xenograft tumor treated with enzalutamide | 2017 | [86] |
| DU145-TxR | Chemo R | DU145 | paclitaxel | culture with paclitaxel | 2007 | [87,88,89,90,91,92,93,94,95,96] |
| PC-3-TxR | Chemo R | PC-3 | paclitaxel | culture with paclitaxel | 2007 | [87,88,89,90,91,92,93,94,95,96] |
| PC-3PR | Chemo R | PC-3 | paclitaxel | culture with paclitaxel | 2010 | [97,98,99,100] |
| PC-3-Pa | Chemo R | PC-3 | paclitaxel | culture with paclitaxel | 2018 | [101] |
| PC-3dR | Chemo R | PC-3 | docetaxel | culture with docetaxel | 2008 | [102] |
| DU145R (O’Neill) | Chemo R | DU145 | docetaxel | culture with docetaxel | 2011 | [103,104] |
| 22Rv1R | Chemo R | 22Rv1 | docetaxel | culture with docetaxel | 2011 | [103,104] |
| PC-3 D12 | Chemo R | PC-3 | docetaxel | culture with docetaxel | 2011 | [103,104] |
| DU145R (Marin) | Chemo R | DU145 | docetaxel | culture with docetaxel | 2012 | [105] |
| PC-3R | Chemo R | PC-3 | docetaxel | culture with docetaxel | 2012 | [105] |
| DU145CR | Chemo R | DU145 | cabazitaxel | culture with cabazitaxel | 2018 | [106] |
| PC-3CR | Chemo R | PC-3 | cabazitaxel | culture with cabazitaxel | 2018 | [106] |
| DU145-TxR/CxR | Chemo R | DU145-TxR | cabazitaxel | culture with cabazitaxel | 2018 | [107] |
| PC-3-TxR/CxR | Chemo R | PC-3-TxR | cabazitaxel | culture with cabazitaxel | 2018 | [107] |
| Name | Pathology | Origin | Host Mouse | Method | First Report Year | References |
|---|---|---|---|---|---|---|
| Rotterdam PC-models | Adeno, NEPC | primary, metastasis | Athymic Nude | SC | 1977 | [22,75,121,125,126,127] |
| LuCaP series | Adeno, NEPC | primary, metastasis | SCID | SC | 1991 | [20,128,129,130,131,132,133,134,135,136,137,138,139,140] |
| LAPC-series | Adeno | metastasis | SCID | SC | 1997 | [63,141] |
| BM18 | Adeno | metastasis | SCID | SC | 2005 | [142] |
| LTL-series | Adeno, NEPC | primary, metastasis | NOD/SCID | SR | 2008 | [143,144,145,146,147,148,149,150] |
| KuCaP-2 | Adeno | local recurrent | Athymic Nude | SC | 2010 | [151] |
| MDA Pca series | Adeno, NEPC | primary, metastasis | SCID | SC | 2011 | [152,153,154,155,156,157] |
| Monash University PDX series | Adeno, NEPC | primary, metastasis | NOD/SCID, NSG | SR | 2011 | [124,158,159,160,161] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells. Cells 2019, 8, 74. https://doi.org/10.3390/cells8010074
Namekawa T, Ikeda K, Horie-Inoue K, Inoue S. Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells. Cells. 2019; 8(1):74. https://doi.org/10.3390/cells8010074
Chicago/Turabian StyleNamekawa, Takeshi, Kazuhiro Ikeda, Kuniko Horie-Inoue, and Satoshi Inoue. 2019. "Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells" Cells 8, no. 1: 74. https://doi.org/10.3390/cells8010074
APA StyleNamekawa, T., Ikeda, K., Horie-Inoue, K., & Inoue, S. (2019). Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells. Cells, 8(1), 74. https://doi.org/10.3390/cells8010074