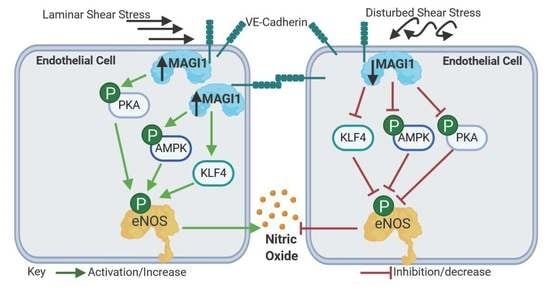
MAGI1 Mediates eNOS Activation and NO Production in Endothelial Cells in Response to Fluid Shear Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Antibodies
2.3. Cell Culture
2.4. Immunofluorescence Staining and Confocal Microscopy
2.5. Plasmids, Lentivirus Production and Transduction
2.6. Fluid Shear Stress Experiments
2.7. Real-Time PCR
2.8. Western Blotting
2.9. NO Measurement
2.10. Kinase Inhibition Assays
2.11. Animal Studies
2.12. Isolation of Mouse Lung Endothelial Cells
2.13. Immunohistochemical Staining
2.14. Statistical Analysis
3. Results
3.1. MAGI1 Localizes at Endothelial Cell-Cell Contacts and its Expression is Induced by Fluid Shear Stress
3.2. MAGI1 Facilitates Endothelial Cell Alignment and KLF4 Expression in Response to Fluid Shear Stress
3.3. MAGI1 Promotes eNOS Phosphorylation and NO Production in Response to Fluid Shear Stress
3.4. MAGI1-Induced eNOS Phosphorylation at Ser1177 is Independent of AKT and CaMKII
3.5. AMPK and PKA Mediate MAGI1-Induced eNOS Ser1177 Phosphorylation
3.6. Transgenic Endothelial Cell Expression of MAGI1 Induces eNOS Phosphorylation at Ser1177 and Increases NO Production in Endothelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J. Structure-function aspects in the nitric oxide synthases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 339–359. [Google Scholar] [CrossRef]
- Feletou, M.; Kohler, R.; Vanhoutte, P.M. Nitric oxide: Orchestrator of endothelium-dependent responses. Ann. Med. 2012, 44, 694–716. [Google Scholar] [CrossRef] [PubMed]
- Sessa, W.C. eNOS at a glance. J. Cell Sci. 2004, 117, 2427–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Fulton, D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Front. Physiol. 2013, 4, 347. [Google Scholar] [CrossRef] [PubMed]
- Dudzinski, D.M.; Igarashi, J.; Greif, D.; Michel, T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 235–276. [Google Scholar] [CrossRef]
- Corson, M.A.; James, N.L.; Latta, S.E.; Nerem, R.M.; Berk, B.C.; Harrison, D.G. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ. Res. 1996, 79, 984–991. [Google Scholar] [CrossRef]
- Atochin, D.N.; Huang, P.L. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflug. Arch. 2010, 460, 965–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulton, D.; Gratton, J.P.; McCabe, T.J.; Fontana, J.; Fujio, Y.; Walsh, K.; Franke, T.F.; Papapetropoulos, A.; Sessa, W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999, 399, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.P.; Mitchelhill, K.I.; Michell, B.J.; Stapleton, D.; Rodriguez-Crespo, I.; Witters, L.A.; Power, D.A.; Ortiz de Montellano, P.R.; Kemp, B.E. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999, 443, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Fleming, I.; Fisslthaler, B.; Dimmeler, S.; Kemp, B.E.; Busse, R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 2001, 88, E68–E75. [Google Scholar] [CrossRef] [PubMed]
- Michell, B.J.; Chen, Z.; Tiganis, T.; Stapleton, D.; Katsis, F.; Power, D.A.; Sim, A.T.; Kemp, B.E. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J. Biol. Chem. 2001, 276, 17625–17628. [Google Scholar] [CrossRef]
- Feng, X.; Jia, S.; Martin, T.A.; Jiang, W.G. Regulation and involvement in cancer and pathological conditions of MAGI1, a tight junction protein. Anticancer Res. 2014, 34, 3251–3256. [Google Scholar]
- Laura, R.P.; Ross, S.; Koeppen, H.; Lasky, L.A. MAGI-1: A widely expressed, alternatively spliced tight junction protein. Exp. Cell Res. 2002, 275, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Chastre, E.; Abdessamad, M.; Kruglov, A.; Bruyneel, E.; Bracke, M.; Di Gioia, Y.; Beckerle, M.C.; van Roy, F.; Kotelevets, L. TRIP6, a novel molecular partner of the MAGI-1 scaffolding molecule, promotes invasiveness. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Fukuhara, S.; Yamagishi, A.; Sako, K.; Kamioka, Y.; Masuda, M.; Nakaoka, Y.; Mochizuki, N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol. Biol. Cell 2006, 17, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Ishida, T.; Kuriyama, M.; Hirata, K.; Hayashi, Y. Interaction of endothelial cell-selective adhesion molecule and MAGI-1 promotes mature cell-cell adhesion via activation of RhoA. Genes Cells Devoted Mol Cell. Mech. 2010, 15, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Filipovic, N.; Ghimire, K.; Saveljic, I.; Milosevic, Z.; Ruegg, C. Computational modeling of shear forces and experimental validation of endothelial cell responses in an orbital well shaker system. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Ashpole, N.E.; Overby, D.R.; Ethier, C.R.; Stamer, W.D. Shear stress-triggered nitric oxide release from Schlemm’s canal cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8067–8076. [Google Scholar] [CrossRef]
- Zaric, J.; Joseph, J.M.; Tercier, S.; Sengstag, T.; Ponsonnet, L.; Delorenzi, M.; Ruegg, C. Identification of MAGI1 as a tumor-suppressor protein induced by cyclooxygenase-2 inhibitors in colorectal cancer cells. Oncogene 2012, 31, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Schnittler, H.J.; Franke, R.P.; Akbay, U.; Mrowietz, C.; Drenckhahn, D. Improved in vitro rheological system for studying the effect of fluid shear stress on cultured cells. Am. J. Physiol. 1993, 265, C289–C298. [Google Scholar] [CrossRef]
- Buschmann, M.H.; Dieterich, P.; Adams, N.A.; Schnittler, H.J. Analysis of flow in a cone-and-plate apparatus with respect to spatial and temporal effects on endothelial cells. Biotechnol. Bioeng. 2005, 89, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Seebach, J.; Donnert, G.; Kronstein, R.; Werth, S.; Wojciak-Stothard, B.; Falzarano, D.; Mrowietz, C.; Hell, S.W.; Schnittler, H.J. Regulation of endothelial barrier function during flow-induced conversion to an arterial phenotype. Cardiovasc. Res. 2007, 75, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Seebach, J.; Dieterich, P.; Luo, F.; Schillers, H.; Vestweber, D.; Oberleithner, H.; Galla, H.J.; Schnittler, H.J. Endothelial barrier function under laminar fluid shear stress. Lab. Investig. 2000, 80, 1819–1831. [Google Scholar] [CrossRef]
- Levesque, M.J.; Nerem, R.M. The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng. 1985, 107, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Baker, B.M.; Chen, C.S.; Schwartz, M.A. Endothelial cell sensing of flow direction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2130–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarreal, G., Jr.; Zhang, Y.; Larman, H.B.; Gracia-Sancho, J.; Koo, A.; Garcia-Cardena, G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010, 391, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Fisslthaler, B.; Dimmeler, S.; Hermann, C.; Busse, R.; Fleming, I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol. Scand. 2000, 168, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Kotelevets, L.; van Hengel, J.; Bruyneel, E.; Mareel, M.; van Roy, F.; Chastre, E. Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Patrie, K.M.; Drescher, A.J.; Welihinda, A.; Mundel, P.; Margolis, B. Interaction of two actin-binding proteins, synaptopodin and alpha-actinin-4, with the tight junction protein MAGI-1. J. Biol.Chem. 2002, 277, 30183–30190. [Google Scholar] [CrossRef]
- Jalan-Sakrikar, N.; Bartlett, R.K.; Baucum, A.J., 2nd; Colbran, R.J. Substrate-selective and calcium-independent activation of CaMKII by alpha-actinin. J. Biol. Chem. 2012, 287, 15275–15283. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, Y.; Guo, Z.; Li, Y.; Beggs, A.H.; Liao, J.K. Dynamic regulation of endothelial NOS mediated by competitive interaction with alpha-actinin-4 and calmodulin. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 1450–1457. [Google Scholar] [CrossRef]
- Zhang, Q.J.; McMillin, S.L.; Tanner, J.M.; Palionyte, M.; Abel, E.D.; Symons, J.D. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: Role of vascular signalling kinases. J. Physiol. 2009, 587, 3911–3920. [Google Scholar] [CrossRef]
- Boo, Y.C.; Sorescu, G.; Boyd, N.; Shiojima, I.; Walsh, K.; Du, J.; Jo, H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: Role of protein kinase A. J. Biol. Chem. 2002, 277, 3388–3396. [Google Scholar] [CrossRef]
- Sun, J.F.; Phung, T.; Shiojima, I.; Felske, T.; Upalakalin, J.N.; Feng, D.; Kornaga, T.; Dor, T.; Dvorak, A.M.; Walsh, K.; et al. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc. Natl. Acad. Sci. USA 2005, 102, 128–133. [Google Scholar] [CrossRef]
- Gregorc, U.; Ivanova, S.; Thomas, M.; Guccione, E.; Glaunsinger, B.; Javier, R.; Turk, V.; Banks, L.; Turk, B. Cleavage of MAGI-1, a tight junction PDZ protein, by caspases is an important step for cell-cell detachment in apoptosis. Apoptosis 2007, 12, 343–354. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Seabold, G.K.; Horak, M.; Petralia, R.S. MAGUKs, synaptic development, and synaptic plasticity. Neuroscientist 2011, 17, 493–512. [Google Scholar] [CrossRef]
- Wegmann, F.; Ebnet, K.; Du Pasquier, L.; Vestweber, D.; Butz, S. Endothelial adhesion molecule ESAM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp. Cell Res. 2004, 300, 121–133. [Google Scholar] [CrossRef]
- Zmajkovicova, K.; Jesenberger, V.; Catalanotti, F.; Baumgartner, C.; Reyes, G.; Baccarini, M. MEK1 is required for PTEN membrane recruitment, AKT regulation, and the maintenance of peripheral tolerance. Mol. Cell 2013, 50, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Rigothier, C.; Auguste, P.; Welsh, G.I.; Lepreux, S.; Deminiere, C.; Mathieson, P.W.; Saleem, M.A.; Ripoche, J.; Combe, C. IQGAP1 interacts with components of the slit diaphragm complex in podocytes and is involved in podocyte migration and permeability in vitro. PLoS ONE 2012, 7, e37695. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.F.; Waaijenborg, S.; Crapo, J.D.; Bowler, R.P.; Akkerman, J.W.; Slot, J.W. Colocalization of eNOS and the catalytic subunit of PKA in endothelial cell junctions: A clue for regulated NO production. J. Histochem. Cytochem. 2004, 52, 1277–1285. [Google Scholar] [CrossRef]
- Turnham, R.E.; Scott, J.D. Protein kinase A catalytic subunit isoform PRKACA.; History, function and physiology. Gene 2016, 577, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Emery, A.C.; Eiden, M.V.; Mustafa, T.; Eiden, L.E. Rapgef2 connects GPCR-mediated cAMP signals to ERK activation in neuronal and endocrine cells. Sci. Signal. 2013, 6, 51. [Google Scholar] [CrossRef]
- Ohnesorge, N.; Viemann, D.; Schmidt, N.; Czymai, T.; Spiering, D.; Schmolke, M.; Ludwig, S.; Roth, J.; Goebeler, M.; Schmidt, M. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4). J. Biol. Chem. 2010, 285, 26199–26210. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Jimenez, J.M.; Ou, K.; McCormick, M.E.; Zhang, L.D.; Davies, P.F. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circ. Res. 2014, 115, 32–43. [Google Scholar] [CrossRef]
- Yoshida, T.; Hayashi, M. Role of Kruppel-like factor 4 and its binding proteins in vascular disease. J. Atheroscler. Thromb. 2014, 21, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamashita, M.; Hayashi, M. Kruppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells. J. Biol. Chem. 2012, 287, 25706–25714. [Google Scholar] [CrossRef] [PubMed]
- Isenovic, E.R.; Soskic, S.; Dungen, H.D.; Dobutovic, B.; Elvis, T.; Simone, I.; Marche, P. Regulation of Endothelial Nitric Oxide Synthase in Pathophysiological Conditions. Cardiovasc. Hematol. Disord. Drug Targets 2011, 11, 109–118. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, K.; Zaric, J.; Alday-Parejo, B.; Seebach, J.; Bousquenaud, M.; Stalin, J.; Bieler, G.; Schnittler, H.-J.; Rüegg, C. MAGI1 Mediates eNOS Activation and NO Production in Endothelial Cells in Response to Fluid Shear Stress. Cells 2019, 8, 388. https://doi.org/10.3390/cells8050388
Ghimire K, Zaric J, Alday-Parejo B, Seebach J, Bousquenaud M, Stalin J, Bieler G, Schnittler H-J, Rüegg C. MAGI1 Mediates eNOS Activation and NO Production in Endothelial Cells in Response to Fluid Shear Stress. Cells. 2019; 8(5):388. https://doi.org/10.3390/cells8050388
Chicago/Turabian StyleGhimire, Kedar, Jelena Zaric, Begoña Alday-Parejo, Jochen Seebach, Mélanie Bousquenaud, Jimmy Stalin, Grégory Bieler, Hans-Joachim Schnittler, and Curzio Rüegg. 2019. "MAGI1 Mediates eNOS Activation and NO Production in Endothelial Cells in Response to Fluid Shear Stress" Cells 8, no. 5: 388. https://doi.org/10.3390/cells8050388
APA StyleGhimire, K., Zaric, J., Alday-Parejo, B., Seebach, J., Bousquenaud, M., Stalin, J., Bieler, G., Schnittler, H. -J., & Rüegg, C. (2019). MAGI1 Mediates eNOS Activation and NO Production in Endothelial Cells in Response to Fluid Shear Stress. Cells, 8(5), 388. https://doi.org/10.3390/cells8050388