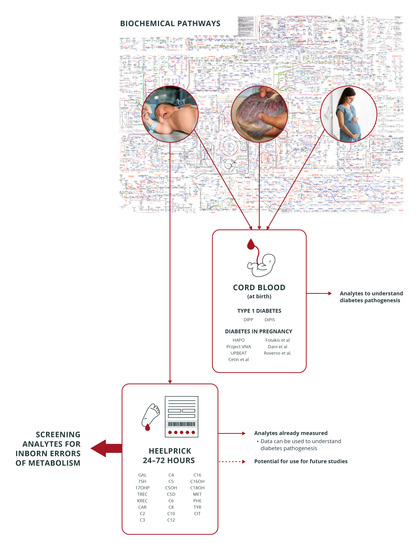
Newborn Screening Samples for Diabetes Research: An Underused Resource
Abstract
:1. Introduction
2. A Brief History of Newborn Screening
3. Available Literature on Diabetes and Newborn Screening
4. Studies Using Cord Blood in Diabetes Research Evaluating Metabolite Derangements
5. Newborn Screening Dried Blood Spots vs. Cord Blood in Diabetes Research: Advantages and Disadvantages as a Testing Sample
5.1. Sample Volume and Storage
5.2. Time of Sampling
5.3. Analytes Assessed
6. Examples of How Cord Blood Studies in Diabetes and Newborn Screening Studies May Complement Each Other
7. Considerations in Using Newborn Screening Results for Diabetes Research
8. Beyond Inborn Errors of Metabolism: Other Biomarkers in Newborn Screening
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saudebray, J.-M.; Berghe, G.v.d.; Walter, J.H. Inborn Metabolic Diseases: Diagnosis and Treatment; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Goetzman, E.S.; Gong, Z.; Schiff, M.; Wang, Y.; Muzumdar, R.H. Metabolic pathways at the crossroads of diabetes and inborn errors. J. Inherit. Metab. Dis. 2018, 41, 5–17. [Google Scholar]
- La Torre, D.; Seppänen-Laakso, T.; Larsson, H.E.; Hyotylainen, T.; Sten, A.; Ivarsson, S.A.; Lernmark, A.; Orešič, M.; DiPiS Study Grp. Decreased cord-blood phospholipids in young age-at-onset type 1 diabetes. Diabetes 2013, 62, 3951–3956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinkovic, T.; Hyotylainen, T.; Mattila, I.; Poho, P.; Knip, M.; Oresic, M. Altered lipid metabolism preceding onset of islet autoimmunity and type 1 diabetes. Diabetologia 2015, 58, S150. [Google Scholar]
- Orešič, M.; Gopalacharyulu, P.; Mykkänen, J.; Lietzen, N.; Mäkinen, M.; Nygren, H.; Simell, S.; Simell, V.; Hyöty, H.; Veijola, R.; et al. Cord serum lipidome in prediction of islet autoimmunity and type 1 diabetes. Diabetes 2013, 62, 3268–3274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oresic, M.; Simell, S.; Sysi-Aho, M.; Nanto-Salonen, K.; Seppanen-Laakso, T.; Parikka, V.; Katajamaa, M.; Hekkala, A.; Mattila, I.; Keskinen, P.; et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J. Exp. Med. 2008, 205, 2975–2984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflueger, M.; Seppänen-Laakso, T.; Suortti, T.; Hyötyläinen, T.; Achenbach, P.; Bonifacio, E.; Orešič, M.; Ziegler, A.G. Age- and islet autoimmunity-associated differences in amino acid and lipid metabolites in children at risk for type 1 diabetes. Diabetes 2011, 60, 2740–2747. [Google Scholar] [CrossRef] [Green Version]
- Dani, C.; Bresci, C.; Berti, E.; Ottanelli, S.; Mello, G.; Mecacci, F.; Breschi, R.; Hu, X.; Tenori, L.; Luchinat, C. Metabolomic profile of term infants of gestational diabetic mothers. J. Matern.-Fetal Neonatal Med. 2014, 27, 537–542. [Google Scholar] [CrossRef]
- Cetin, I.; de Santis, M.S.; Taricco, E.; Radaelli, T.; Teng, C.; Ronzoni, S.; Spada, E.; Milani, S.; Pardi, G. Maternal and fetal amino acid concentrations in normal pregnancies and in pregnancies with gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2005, 192, 610–617. [Google Scholar] [CrossRef]
- Fotakis, C.; Zoga, M.; Baskakis, C.; Tsiaka, T.; Boutsikou, T.; Briana, D.D.; Dendrinou, K.; Malamitsi-Puchner, A.; Zoumpoulakis, P. Investigating the metabolic fingerprint of term infants with normal and increased fetal growth. RSC Adv. 2016, 6, 79325–79334. [Google Scholar] [CrossRef]
- Lowe, W.L.; Bain, J.R., Jr.; Nodzenski, M.; Reisetter, A.C.; Muehlbauer, M.J.; Stevens, R.D.; Ilkayeva, O.R.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; et al. Maternal BMI and glycemia impact the fetal metabolome. Diabetes Care 2017, 40, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Perng, W.; Rifas-Shimanb, S.L.; McCullochc, S.; Chatzid, L.; Mantzorosg, C.; Hivert, M.-F.; Oken, E. Associations of cord blood metabolites with perinatal characteristics, newborn anthropometry, and cord blood hormones in project viva. Metab. Clin. Exp. 2017, 76, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Hellmuth, C.; Uhl, O.; Godfrey, K.; Briley, A.; Welsh, P.; Pasupathy, D.; Seed, P.T.; Koletzko, B.; Poston, L. Cord metabolic profiles in obese pregnant women: Insights into offspring growth and body composition. J. Clin. Endocrinol. Metab. 2018, 103, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Roverso, M.; Di Marco, V.; Badocco, D.; Pastore, P.; Calanducci, M.; Cosmi, E.; Visentin, S. Maternal, placental and cordonal metallomic profiles in gestational diabetes mellitus. Metallomics 2019, 11, 676–685. [Google Scholar] [CrossRef]
- Kadakia, R.; Scholtens, D.M.; Rouleau, G.W.; Talbot, O.; Ilkayeva, O.R.; George, T.; Josefson, J.L. Cord blood metabolites associated with newborn adiposity and hyperinsulinemia. J. Pediatr. 2018, 203, 144. [Google Scholar] [CrossRef]
- Walter, J.H.; Patterson, A.; Till, J.; Besley, G.T.N.; Fleming, G.; Henderson, M.J. Bloodspot acylcarnitine and amino acid analysis in cord blood samples: Efficacy and reference data from a large cohort study. J. Inherit. Metab. Dis. 2009, 32, 95–101. [Google Scholar] [CrossRef]
- Wilcken, B.; Wiley, V. Newborn screening. Pathology 2008, 40, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Blau, N.; Shen, N.; Carducci, C. Molecular genetics and diagnosis of phenylketonuria: State of the art, expert review of molecular. Diagnostics 2014, 14, 655–671. [Google Scholar] [CrossRef]
- Norman, R.; Haas, M.; Chaplin, M.; Joy, P.; Wilcken, B. Economic evaluation of tandem mass spectrometry newborn screening in Australia. Pediatrics 2009, 123, 451–457. [Google Scholar] [CrossRef]
- Newborn Blood Spot Test. Available online: https://www.nhs.uk/conditions/pregnancy-and-baby/newborn-blood-spot-test (accessed on 1 May 2020).
- Downing, M.; Pollitt, R. Newborn bloodspot screening in the UK--past, present and future. Ann. Clin. Biochem. 2008, 45, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Kyvsgaard, J.; Overgaard, A.J.; Thorsen, S.U.; Hansen, T.H.; Pipper, C.B.; Mortensen, H.B.; Pociot, F.; Svensson, J. High neonatal blood iron content is associated with the risk of childhood type 1 diabetes mellitus. Nutrients 2017, 9, 1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, T.J.; Besser, R.E.; Perry, M.; Babiker, T.; Knight, B.A.; Shepherd, M.H.; Ellard, S.; Flanagan, S.E.; Hattersley, A.T. Screening for neonatal diabetes at day 5 of life using dried blood spot glucose measurement. Diabetologia 2017, 60, 2168–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadario, F.; Savastio, S.; Pagliardini, V.; Bagnati, M.; Vidali, M.; Cerutti, F.; Rabbone, I.; Fontana, F.; Lera, R.; De Donno, V.; et al. Vitamin D levels at birth and risk of type 1 diabetes in childhood: A case–control study. Acta Diabetol. 2015, 52, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, R.; Thorsen, S.U.; Cohen, A.S.; Lundqvist, M.; Frederiksen, P.; Pipper, C.B.; Pociot, F.; Thygesen, L.C.; Ascherio, A.; Svensson, J.; et al. Neonatal vitamin D status is not associated with later risk of type 1 diabetes: Results from two large Danish population-based studies. Diabetologia 2016, 59, 1871–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyvsgaard, J.N.; Overgaard, A.J.; Jacobsen, L.D.; Thorsen, S.U.; Pipper, C.B.; Hansen, T.H.; Husted, S.; Mortensen, H.B.; Pociot, F.; Svensson, J. Low perinatal zinc status is not associated with the risk of type 1 diabetes in children. Pediatr. Diabetes 2016, 18, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Marca, G.L.; Malvagia, S.; Toni, S.; Piccini, B.; Di Ciommo, V.; Bottazzo, G.F. Children who develop type 1 diabetes early in life show low levels of carnitine and amino acids at birth: Does this finding shed light on the etiopathogenesis of the disease? Nutr. Diabetes 2013, 3, e94. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pintos, P.; de Castro, M.J.; Roca, I.; Rite, S.; Lopez, M.; Couce, M.L. Similarities between acylcarnitine profiles in large for gestational age newborns and obesity. Sci. Rep. 2017, 7, 16267. [Google Scholar] [CrossRef]
- Simaite, D.; Kofent, J.; Gong, M.; Rüschendorf, F.; Jia, S.; Arn, P.; Bentler, K.; Ellaway, C.; Kühnen, P.; Hoffmann, G.F.; et al. Recessive mutations in PCBD1 cause a new type of early-onset diabetes. Diabetes 2014, 63, 3557–3564. [Google Scholar] [CrossRef] [Green Version]
- Eising, S.; Svensson, J.; Skogstrand, K.; Nilsson, A.; Lynch, K.; Andersen, P.S.; Lernmark, Å.; Hougaard, D.M.; Pociot, F.; Nørgaard-Pedersen, B.; et al. Type 1 diabetes risk analysis on dried blood spot samples from population-based newborns: Design and feasibility of an unselected case-control study. Paediatr. Perinat. Epidemiol. 2007, 21, 507–517. [Google Scholar] [CrossRef]
- Nguyen, Q.C.; Whitsel, E.A.; Tabor, J.W.; Cuthbertson, C.C.; Wener, M.H.; Potter, A.J.; Halpern, C.T.; Killeya-Jones, L.A.; Hussey, J.M.; Suchindran, C.; et al. Blood spot-based measures of glucose homeostasis and diabetes prevalence in a nationally representative population of young US adults. Ann. Epidemiol. 2014, 24, 903. [Google Scholar] [CrossRef] [Green Version]
- Pollock, A.J.; Allen, D.B.; Wiebe, D.; Eickhoff, J.; MacDonald, M.; Baker, M. Development of filter paper hemoglobin A1c assay applicable to newborn screening. Clin. Chim. Acta 2016, 457, 24–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aburuz, S.; Millership, J.; McElnay, J. Dried blood spot liquid chromatography assay for therapeutic drug monitoring of metformin. J. Chromatogr. B. 2006, 832, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Bakhireva, L.N.; Sharkis, J.; Shrestha, S.; Miranda-Sohrabji, T.J.; Williams, S.; Miranda, R.C. Prevalence of prenatal alcohol exposure in the State of texas as assessed by phosphatidylethanol in Newborn Dried blood spot specimens. Alcohol. Clin. Exp. Res. 2017, 41, 1004–1011. [Google Scholar] [CrossRef]
- Basu, N.; Eng, J.W.L.; Perkins, M.; Santa-Rios, A.; Martincevic, G.; Carlson, K.; Neitzel, R.L. Development and application of a novel method to characterize methylmercury exposure in newborns using dried blood spots. Environ. Res. 2017, 159, 276–282. [Google Scholar] [CrossRef] [PubMed]
- McCabe, E.R.; Huang, S.Z.; Seltzer, W.K.; Law, M.L. DNA microextraction from dried blood spots on filter paper blotters: Potential applications to newborn screening. Hum. Genet. 1987, 75, 213–216. [Google Scholar] [CrossRef]
- Harper, P.; Wadström, C.; Cederblad, G. Carnitine measurements in liver, muscle tissue, and blood in normal subjects. Clin. Chem. 1993, 39, 592–599. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas teB, Belgium: International Diabetes Federation hwdo; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- De Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, K.; Wotton, T.; Wiley, V. The evolution of blood-spot newborn screening. Transl. Pediatr. 2014, 3, 63–70. [Google Scholar]
- Fell, D.B.; Hawken, S.; Wong, C.A.; Wilson, L.A.; Murphy, M.S.Q.; Chakraborty, P.; Lacaze-Masmonteil, T.; Potter, B.K.; Wilson, K. Using newborn screening analytes to identify cases of neonatal sepsis. Sci. Rep. 2017, 7, 18020. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.; Hawken, S.; Potter, B.K.; Chakraborty, P.; Walker, M.; Ducharme, R.; Little, J. Accurate prediction of gestational age using newborn screening analyte data. Am. J. Obstet. Gynecol. 2016, 214, 513.e1–513.e9. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, K.G.; Kastenberg, Z.J.; Moss, R.L.; Enns, G.M.; Cowan, T.M.; Shaw, G.M.; Stevenson, D.K.; Sinclair, T.J.; Scharfe, C.; Ryckman, K.K.; et al. Acylcarnitine profiles reflect metabolic vulnerability for necrotizing enterocolitis in newborns born premature. J. Pediatrics 2017, 181, 80. [Google Scholar] [CrossRef] [Green Version]
- Horgan, R.P.; Broadhurst, D.I.; Walsh, S.K.; Dunn, W.B.; Brown, M.; Roberts, C.T.; North, R.A.; McCowan, L.M.; Kell, D.B.; Baker, P.N.; et al. Metabolic profiling uncovers a phenotypic signature of small for gestational age in early pregnancy. J. Proteome Res. 2011, 10, 3660–3673. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, K.K.; Berberich, S.L.; Dagle, J.M. Predicting gestational age using neonatal metabolic markers. Am. J. Obstet. Gynecol. 2016, 214, 515.e1–515.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelliffe-Pawlowski, L.L.; Norton, M.E.; Baer, R.J.; Santos, N.; Rutherford, G.W. Gestational dating by metabolic profile at birth: A California cohort study. Am. J. Obstet. Gynecol. 2016, 214, 511.e1–511.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusconi, B.; Warner, B.B. The hidden treasure of neonatal screening: Identifying new risk factors and possible mechanisms of necrotizing enterocolitis through big data. J. Pediatrics 2017, 181, 9–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornel, M.; Rigter, T.; Weinreich, S. Newborn screening in Europe: Expert opinion document. Available online: http://www.iss.it/cnmr/index.php?lang=1&id=1621&tipo=72 (accessed on 30 April 2020).
- Aisa, M.C.; Cappuccini, B.; Barbati, A.; Clerici, G.; Torlone, E.; Gerli, S.; Di Renzo, G.C. Renal Consequences of Gestational Diabetes Mellitus in Term Neonates: A Multidisciplinary Approach to the DOHaD Perspective in the Prevention and Early Recognition of Neonates of GDM Mothers at Risk of Hypertension and Chronic Renal Diseases in Later Life. J. Clin. Med. 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrat, L.A.; Vehik, K.; Sharp, S.A.; Lernmark, Å.; Rewers, M.J.; She, J.X.; Ziegler, A.G.; Toppari, J.; Akolkar, B.; Krischer, J.P.; et al. A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat. Med. 2020, 26, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Gárate-Escamilla, A.K.; Garza-Padilla, E.; Carvajal Rivera, A.; Salas-Castro, C.; Andrès, E.; Hajjam, E.L.; Hassani, A. Cluster Analysis: A New Approach for Identification of Underlying Risk Factors and Demographic Features of First Trimester Pregnancy Women. J. Clin. Med. 2020, 9, 2247. [Google Scholar] [CrossRef]
- Guthrie, R. The introduction of newborn screening for phenylketonuria. A personal history. Eur. J. Pediatr. 1996, 155 (Suppl. 1), S4–S5. [Google Scholar] [CrossRef]
| Study/Year | Cohort/Country | Analysis Method | Metabolite Deranged |
|---|---|---|---|
| Type 1 Diabetes | |||
| Oresic et al., 2008 [5] | DIPP/Finland | UPLC/MS GCxGC-TOF/MS | Phosphatidylcholine (↓) Succinic/citric acid (↓) |
| Oresic et al., 2013 [6] | DiPiS/Sweden | UPLC/MS | Phosphatidylcholine (↓) Succinic/citric acid (↓) |
| La Torre et al., 2013 [3] | DiPiS | UPLC/MS | Phosphatidylcholine (↓) Phosphatidylethanolamine (↓) Triglycerides (↓) |
| Diabetes in Pregnancy | |||
| Cetin et al., 2005 [9] | Infants of GDM mothers/Milano | HPLC | Valine, Methionine, Phenylalanine, Isoleucine, Leucine, Ornithine, Glutamate (↑) Proline, Alanine (↑) Glutamine (↓) |
| Dani et al., 2014 [8] | Infants of GDM mothers/Florence | NMRS | Pyruvate, Histidine, Alanine, Valine, Methionine, Arginine, Lysine, α-ketoisovaleric acid, Hypoxanthine, Lipoprotein, Lipid (↑) Glucose (↓) |
| Fotakis et al., 2016 [10] * | Infants of GDM mothers/Athens | NMRS | Large for gestational age GDM vs. appropriate for gestational age: Valine, leucine, isoleucine, lysine, aCH2, N-acetylglutamine, acetoacetic acid, glutamine/glutamic acid, threonine, creatine and histidine (↑) Large for gestational age GDM vs. large for gestational age non-GDM: glucose, glutamine, valine, histidine, alanine (↑) |
| Lowe et al., 2017 [11] | HAPO/Mexican-Am, Thai, N Europe, Afri-Carib | MS/MS | Maternal BMI: positive association with BCAA and byproducts, Phenylalanine, AC C3, C4, C5 Maternal Fasting Glucose: C4OH (positive association; Mex-Am only) Maternal 1-h Glucose: positive association with 3OH butyrate/AC-C4OH, Glycerol, AC C10-OH/C8DC Maternal Insulin Resistance: positive association with BCAA and derivatives, AC C3, AC C4/Ci4, AC C5, AC C5-DC, AC C4-OH, AC C2, Glycerol, Asparagine/asp (Afri-Car) Subgroup analysis-cord blood c-peptide: negative association with leucine/isoleucine and positive association with AC C5 Newborn Outcomes Birth weight: positive association with Serine, Proline, Glutamine/Glutamate, Glycine, 3OH and AC-C3, C12- OH/C10-OH, C10-OH/C8-DC, C8:1-DC, C6-DC/C8-OH, C8:1-OH/C6:1-DC Negative association with Triglycerides, AC-C20-OH/C18-DC (Thai) Adjusting for cord c-peptide: (in addition to) positive association with Leucine/Isoleucine, Arginine, Ornithine, Citrulline Negative association with AC C4/Ci4, AC-C20-OH/C18-DC (Thai) AC C8:1, AC C10:3 (Afro-Cari) |
| Perng et al., 2017 [12] | Project Viva/Massachusetts | UPLC/MS | No association with BCAA or metabolites of energy production and cell proliferation pathways |
| Patel et al., 2018 [13] | UPBEAT/UK | LC-MS/MS | Adiponectin (↓), Isocitric acid and Lysophosphatidylcholine 18:1 (↑) |
| Roverso et al., 2019 [14] | Infants of GDM mothers/Padua | ICP-MS | Ca, Cu, Na, Zn (↑), Fe, K, Mn, P, Rb, S, and Si (↓) |
| Pharmacotherapy during pregnancy: | |||
| None looking at acylcarnitines or amino acids on cord blood | |||
| Study | Analyte Deranged | Disorder Tested on NBS |
|---|---|---|
| Lowe et al. (2017) [14] | Maternal BMI: Phenylalanine (+ association) AC C3, AC C5, AC C4; Leucine/Isoleucine Cord C-peptide and BW (+association) Arginine Cord C-peptide and SSF AC C4-OH | PKU/Pterin defects Propionic aciduria/methylmalonic aciduria 2-methylbutyrylCoA-dehydrogenase deficiency Isobutyryl CoA-dehydrogenase deficiency Short chain dehydrogenase deficiency Multiple acyl CoA dehydrogenase deficiency Maple syrup urine disease Arginase deficiency Short chain hydroxy acyl CoA dehydrogenase deficiency |
| Kadakia et al. (2018) [15] | Cord C-peptide (−association) Tyrosine BW (+association) AC C10:1 GDM C16 | Tyrosinemia Secondary marker for Medium Chain CoA deficiency Very long chain acyl CoA dehydrogenase deficiency |
| Author/Year Study Design | Study Objective | Population Characteristics | Sample Size | Method of Analysis | Results | Comments |
|---|---|---|---|---|---|---|
| Type 1 Diabetes risk and NBS results only | ||||||
| La Marca, 2013 [7] Case control | To investigate the relationship between carnitines and amino acids with T1DM | 50 children from Tuscany and Umbria with T1DM diagnosed ≤5 years; HLA genotyped; Antibody status checked | 250 neonates’ NBS results Controls: 200 (same analytic batch) | LC-MS/MS | Lower C2, C3, C4, C5, C14, C16, C18, Total and free carnitine Alanine (p ≤ 0.05) | Reported as mean: Total carnitine, acylcarnitine, C2, C5, alanine; Ile and Leu reported as one analyte; CV: none reported |
| Reanalyzing for metabolites and T1DM risk | ||||||
| Cadario, 2015 [8] Case control | To investigate variations in Vitamin D concentrations at birth and the risk of developing T1DM up to 10 years; potential modifier effect of ethnic groups on the association | Piedmont Diabetes Childhood Registry; 67 children with T1DM 0–10 years | 300 neonates’ NBS card 267 controls, matched for birthday (±30 days), place of birth and ethnic group | LC-MS/MS | No association as a whole; 36 cases and 103 controls <2.14; OR 1.76 (0.92–3.38) 31 cases and 133 controls ≥2.14 OR 1.00). | Subgroup analysis: Migrants: 20 cases 31 controls <2.14; OR 14.02 (1.76–111.7); 3 cases 26 controls >2.14; OR 1.0; CV: not reported |
| Jacobsen, 2016 [9] Case-cohort 2 models (with/out HLA matching) | To investigate low levels of 25(OH)D at birth and the risk of developing type 1 diabetes before the age of 18 years | Danish Childhood Diabetes Registry (DanDiabKids) Case control: 912 Case cohort: 2866 | Case-cohort: 3778; Method of choosing controls unspecified; Case control: Model 1–527 pairs Model 2–429 pairs (858 total); These pairs were HLA matched; Controls chosen via DBS card next to index case card | LC-MS | No associations Case cohort: Sub-cohort: (median) 23.8 (15.5, 36.7) cases: 24.3 (14.8, 38.8) Case control: Cases: 21.3 (12.5–33.1) Controls: 21.1 (12.0–32.9) | Both groups used the same registry; overlap with sampling of 4 individuals with T1DM CV: 15% |
| Kyvsgaard, 2016 [10] Population-based case-control | To investigate association between low perinatal zinc status and the risk of T1DM before 16 years | Danish Childhood Diabetes Register (DanDiabKids)199 cases with T1DM | 398 NBS cards 199 controls; Matched by birth year and month | LA-ICP-MS | No association Reference range: 10–19 μmol/L OR: High zinc: 1; Med high zinc: 0.88 (0.41, 1.85) Low zinc: 0.89 (0.40, 1.97) | All samples, negative controls and reference samples analyzed in the same run. Covariates included: Sex, birth year, season, HLADQ1B status, gestational age, birth weight, maternal age at delivery; CV: 15.9% |
| Kyvsgaard, 2017 [11] Case-control | To investigate association between neonatal iron content and the risk of T1DM before 16 years. | Danish Childhood Diabetes Register (DanDiabKids)199 cases with T1DM HLA-DQB1 genotyping | 398 NBS cards 199 controls chosen by consecutive NBS numbers | LA-ICP-MS | Two-fold risk of T1DM with doubling of iron content; Cases (199): 1.80 (0.30); Controls: (199): 1.74 (0.39) OR 2.07 (95% CI) (1.07; 4.00) | All samples analyzed on the same run; After adjusting for confounders (OR 2.55; 1.04; 6.24) CV: 19.3% |
| Metabolic signature of monogenic diabetes on DBS | ||||||
| McDonald, 2017 [1] Case-control | To assess stability of DBS glucose and the diagnostic accuracy of DBS glucose for neonatal diabetes detection | Newborns part of the Exeter Family Study of Childhood Health; Exeter 10,000 project; infants with genetically confirmed neonatal diabetes (11 cases) | 687 infants; 20 volunteers; 170 infants with genetically confirmed neonatal diabetes | UV Spectrometry (manual rate-reaction hexokinase method) | glucose stable in room temp, 4 °C and −20 °C for up to 5 days, stable >14 days in 4 °C and −20 °C; Mean (SD) glucose at day 5 of life: Infants with neonatal diabetes:10.2–>30.0 mmol/L (normal 4.6 mmol (0.7)) | CV: 10.3% (3 mmol/L); 15% (14 mmol/L); NBS performed day 5 of life; 5/11 infants with neonatal diabetes diagnosed before NBS performed |
| Simaite, 2014 [12] Familial linkage, molecular analysis and animal studies | To identify novel diabetes genes | Consanguineous family with nonautoimmune diabetes BIODEF database | Index family 6 families identified through BIODEF | Not specified (usually MS/MS) | Patients with PCBD1 homozygous mutations may have mild transient hyperphenylalaninemia (>120 umol but <360 umol/L) | 2 patients with normal pH levels; Not all patients with homozygous mutations with diabetes; CV not reported |
| Metabolic signature ofdiabetes on NBS | ||||||
| Sanchez-Pintos, 2017 [13] Observational | To characterize postnatal plasma acylcarnitine profiles in a cohort of LGA newborns. To compare acylcarnitine fingerprint of LGA-GDM vs. LGA-NGDM | All infants born in Hospital | Total N = 2514; SGA: 250; AGA: 2018; LGA: 246 Newborns with GDM exposure: 246 (200 on diet, 46 on insulin); LGA-GDM: 42; LGA-NGDM: 204 | MS/MS (derivatized) | For GDM-LGA, Median higher levels of FC, TC, short-chain acylcarnitines incl C3, lower levels of medium and long-chain acylcarnitines-NS | No information on amino acids; No information on degree of diabetes control during pregnancy |
| Sample Characteristics | NBS DBS | Cord Blood | Comments |
|---|---|---|---|
| Components | whole blood | plasma | DBS samples need correcting for hematocrit |
| Volume | 50–75 μL per blood spot | 60–110 mL | Small volume of DBS in NBS limits use in untargeted metabolomic techniques |
| Storage | Once dry, may be stored at room temperature | Needs special storage facilities to keep temperature between −70 to −20 °C. | DBS more prone to measurements of uncertainty such as transport conditions, weather, etc. |
| Timing of collection | At least 24 h following delivery with some countries testing between day 3–5 following delivery | Collected immediately after delivery | Cord blood samples may reflect placental and maternal metabolism while the neonate is receiving a constant supply of nutrition. NBS DBS reflect infants’ metabolism (independent of maternal and placental influence) with periods of fasting in between feeding. |
| Coverage | Near universal in countries that have NBS programs (>99% in NSW, Australia) incorporated into public health | Variable; usually collected as part of a research project or private cord blood banking | Countries with well-established NBS programs within a framework of socialized medicine are able to draw on NBS DBS for uses outside research (i.e., source of DNA for retrospective cascade testing), forensic medicine, and quality assurance programs |
| Metabolic intermediates tested | Acylcarnitines, free and total acylcarnitines, some amino acids, 17 hydroxyprogesterone, thyrotropin, trypsinogen, galactose Conditions tested (and metabolites) vary among programs | Wide spectrum of intermediates may be tested, including phospholipids and their intermediaries, ceramides, intermediaries of various metabolic networks | NBS testing from DBS in NSW includes testing for other conditions (muscular dystrophies, immune deficiency syndromes, cystic fibrosis). Feasibility of antibody testing on DBS for type 1 diabetes has been established. Volume and storage conditions of cord blood allow discovery and identification of metabolic intermediates not previously described. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrella, J.F.G.L.; Immanuel, J.; Wiley, V.; Simmons, D. Newborn Screening Samples for Diabetes Research: An Underused Resource. Cells 2020, 9, 2299. https://doi.org/10.3390/cells9102299
Estrella JFGL, Immanuel J, Wiley V, Simmons D. Newborn Screening Samples for Diabetes Research: An Underused Resource. Cells. 2020; 9(10):2299. https://doi.org/10.3390/cells9102299
Chicago/Turabian StyleEstrella, Jane Frances Grace Lustre, Jincy Immanuel, Veronica Wiley, and David Simmons. 2020. "Newborn Screening Samples for Diabetes Research: An Underused Resource" Cells 9, no. 10: 2299. https://doi.org/10.3390/cells9102299
APA StyleEstrella, J. F. G. L., Immanuel, J., Wiley, V., & Simmons, D. (2020). Newborn Screening Samples for Diabetes Research: An Underused Resource. Cells, 9(10), 2299. https://doi.org/10.3390/cells9102299