Dynamic Characterization of Structural, Molecular, and Electrophysiological Phenotypes of Human-Induced Pluripotent Stem Cell-Derived Cerebral Organoids, and Comparison with Fetal and Adult Gene Profiles
Abstract
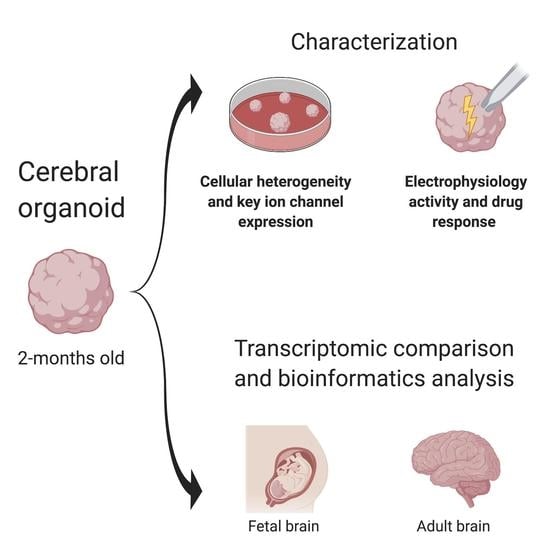
:1. Introduction
2. Methods
2.1. iPSC Culture and 3D Cerebral Organoid Generation from iPSCs
2.2. Immunostaining Analysis of iPSC, Neural Progenitor Cells, Brain Cell-Specific Marker Expression
2.3. Electron Microscopy Analysis of Synapse Structure
2.4. Ribonucleic Acid (RNA) Extraction
2.5. Complementary Deoxyribonucleic Acid (cDNA) Preparation
2.6. qRT-PCR
2.7. Electrophysiology Analysis of Action Potential and Different Channel Activities
2.8. mRNA Expression Profiling
2.9. Ingenuity Pathway Analysis (IPA) Bioinformatic Analysis of mRNA Expression Profiles
2.10. Statistics
3. Results
3.1. iPSCs Are Pluripotent and Differentiate into Cerebral Organoids
3.2. Cerebral Organoids Exhibit Heterogeneous Gene and Protein Expression of the Markers for Different Neural Cell Types, Blood Vessel-Related Smooth Muscle Cells and Endothelial Cells, and Synapses
3.3. Cerebral Organoids Dynamically Develop from iPSCs over Time
3.4. Cerebral Organoids Display Electrical Activity
3.5. Cerebral Organoids Display Various Functional Channel Currents
3.6. Cerebral Organoids Express Gene Profiles Common to Neurodevelopmental Electrophysiological and Signaling Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | adult brain |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| cAMP | cyclic adenosine monophosphate |
| CD31 | cluster of differentiation 31 |
| CO | cerebral organoid |
| CREB | cAMP response element-binding protein |
| cDNA | complementary deoxyribonucleic acid |
| Ct | cycle threshold |
| dNTP | deoxynucleoside triphosphate |
| down | downregulation |
| EGTA | ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| F | fetal brain |
| GABA | gamma-aminobutyric acid |
| IPA | Ingenuity Pathway Analysis |
| GABAA receptor | GABA type A (GABAA) receptor |
| Gapdh | glyceraldehyde 3-phosphate dehydrogenase |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| iPSCs | induced pluripotent stem cells |
| MAP2 | microtubule-associated protein 2 |
| Mark1 | MAP/microtubule affinity-regulating kinase 1 |
| NMDA | N-methyl-d-aspartate |
| ns | no significant difference |
| OCT4 | octamer binding transcription factor 4 |
| PBS | phosphate buffered saline |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| S100B | S100 calcium binding protein B |
| SCN/Nav | voltage-gated sodium channels |
| SMA | smooth muscle cell marker |
| 2D | two-dimensional |
| 3D | three-dimensional |
| up | upregulation |
References
- Slusarz, R.; Jablonska, R.; Krolikowska, A.; Haor, B.; Barczykowska, E.; Biercewicz, M.; Glowacka, M.; Szrajda, J. Measuring scales used for assessment of patients with traumatic brain injury: Multicenter studies. Patient Prefer. Adherence 2015, 9, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, L.; Tyson, S.F. Screening for cognitive impairment after stroke: A systematic review of psychometric properties and clinical utility. J. Rehabil. Med. 2015, 47, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.Y.; Weick, J.P.; Yu, J.; Ma, L.X.; Zhang, X.Q.; Thomson, J.A.; Zhang, S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordoni, M.; Rey, F.; Fantini, V.; Pansarasa, O.; Di Giulio, A.M.; Carelli, S.; Cereda, C. From Neuronal Differentiation of iPSCs to 3D Neuro-Organoids: Modelling and Therapy of Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 3972. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Jiang, H.; Zhang, B.; Feng, J. Modeling Parkinson’s Disease Using Patient-specific Induced Pluripotent Stem Cells. J. Parkinsons Dis. 2018, 8, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Stoddard-Bennett, T.; Reijo Pera, R. Treatment of Parkinson’s Disease through Personalized Medicine and Induced Pluripotent Stem Cells. Cells 2019, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Logan, S.; Arzua, T.; Canfield, S.G.; Seminary, E.R.; Sison, S.L.; Ebert, A.D.; Bai, X. Studying human neurological disorders using induced pluripotent stem cells: From 2D monolayer to 3D organoid and blood brain barrier models. Compr. Physiol. 2019, 9, 565–611. [Google Scholar] [CrossRef]
- Shi, Y.; Kirwan, P.; Smith, J.; Robinson, H.P.; Livesey, F.J. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat. Neurosci. 2012, 15, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Kayama, T.; Okamoto, K.; Gao, M.; Ikegaya, Y.; Sasaki, T. Immature electrophysiological properties of human-induced pluripotent stem cell-derived neurons transplanted into the mouse cortex for 7 weeks. Neuroreport 2019, 30, 169–173. [Google Scholar] [CrossRef]
- Liu, C.; Oikonomopoulos, A.; Sayed, N.; Wu, J.C. Modeling human diseases with induced pluripotent stem cells: From 2D to 3D and beyond. Development 2018, 145. [Google Scholar] [CrossRef] [Green Version]
- Israel, M.A.; Goldstein, L.S. Capturing Alzheimer’s disease genomes with induced pluripotent stem cells: Prospects and challenges. Genome Med. 2011, 3, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israel, M.A.; Yuan, S.H.; Bardy, C.; Reyna, S.M.; Mu, Y.; Herrera, C.; Hefferan, M.P.; Van Gorp, S.; Nazor, K.L.; Boscolo, F.S.; et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 2012, 482, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [Green Version]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Jacob, F.; Song, H.; Ming, G.L. Using brain organoids to understand Zika virus-induced microcephaly. Development 2017, 144, 952–957. [Google Scholar] [CrossRef] [Green Version]
- Camp, J.G.; Badsha, F.; Florio, M.; Kanton, S.; Gerber, T.; Wilsch-Brauninger, M.; Lewitus, E.; Sykes, A.; Hevers, W.; Lancaster, M.; et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 2015, 112, 15672–15677. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, C.; Bienengraeber, M.; Canfield, S.; Koopmeiner, A.; Schafer, R.; Bosnjak, Z.J.; Bai, X. Comparison of cardiomyocyte differentiation potential between type 1 diabetic donor- and nondiabetic donor-derived induced pluripotent stem cells. Cell Transplant. 2015, 24, 2491–2504. [Google Scholar] [CrossRef] [Green Version]
- Horikoshi, Y.; Yan, Y.; Terashvili, M.; Wells, C.; Horikoshi, H.; Fujita, S.; Bosnjak, Z.J.; Bai, X. Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells 2019, 8, 1095. [Google Scholar] [CrossRef] [Green Version]
- Logan, S.; Jiang, C.; Yan, Y.; Inagaki, Y.; Arzua, T.; Bai, X. Propofol alters long non-coding RNA profiles in the neonatal mouse hippocampus: Implication of novel mechanisms in anesthetic-induced developmental neurotoxicity. Cell. Physiol. Biochem. 2018, 49, 2496–2510. [Google Scholar] [CrossRef]
- Han, S.; Yu, F.H.; Schwartz, M.D.; Linton, J.D.; Bosma, M.M.; Hurley, J.B.; Catterall, W.A.; de la Iglesia, H.O. Na(V)1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms. Proc. Natl. Acad. Sci. USA 2012, 109, E368–E377. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Han, C.; Estacion, M.; Vasylyev, D.; Hoeijmakers, J.G.; Gerrits, M.M.; Tyrrell, L.; Lauria, G.; Faber, C.G.; Dib-Hajj, S.D.; et al. Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain 2014, 137, 1627–1642. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.K.; Matsubayashi, M.; Sakaguchi, Y.M.; Hayashi, R.K.; Zheng, C.; Sugie, K.; Hasegawa, M.; Nakagawa, T.; Mori, E. Six-month cultured cerebral organoids from human ES cells contain matured neural cells. Neurosci. Lett. 2018, 670, 75–82. [Google Scholar] [CrossRef]
- Yakoub, A.M.; Sadek, M. Development and Characterization of Human Cerebral Organoids: An Optimized Protocol. Cell. Transplant. 2018, 27, 393–406. [Google Scholar] [CrossRef]
- Noden, D.M. Embryonic origins and assembly of blood vessels. Am. Rev. Respir. Dis. 1989, 140, 1097–1103. [Google Scholar] [CrossRef]
- Munno, D.W.; Syed, N.I. Synaptogenesis in the CNS: An odyssey from wiring together to firing together. J. Physiol. 2003, 552, 1–11. [Google Scholar] [CrossRef]
- Yakoub, A.M.; Sadek, M. Analysis of Synapses in Cerebral Organoids. Cell. Transplant. 2019, 28, 1173–1182. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, F.; Lent, R.; Herculano-Houzel, S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc. Natl. Acad. Sci. USA 2009, 106, 14108–14113. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yan, Y.; Inagaki, Y.; Logan, S.; Bosnjak, Z.J.; Bai, X. Insufficient astrocyte-derived brain-derived neurotrophic factor contributes to propofol-induced neuron death through Akt/glycogen synthase kinase 3beta/mitochondrial fission pathway. Anesth. Analg. 2017, 125, 241–254. [Google Scholar] [CrossRef]
- Watanabe, M.; Buth, J.E.; Vishlaghi, N.; de la Torre-Ubieta, L.; Taxidis, J.; Khakh, B.S.; Coppola, G.; Pearson, C.A.; Yamauchi, K.; Gong, D.; et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell. Rep. 2017, 21, 517–532. [Google Scholar] [CrossRef]
- Hartfield, E.M.; Yamasaki-Mann, M.; Ribeiro Fernandes, H.J.; Vowles, J.; James, W.S.; Cowley, S.A.; Wade-Martins, R. Physiological characterisation of human iPS-derived dopaminergic neurons. PLoS ONE 2014, 9, e87388. [Google Scholar] [CrossRef]
- Monzel, A.S.; Smits, L.M.; Hemmer, K.; Hachi, S.; Moreno, E.L.; van Wuellen, T.; Jarazo, J.; Walter, J.; Bruggemann, I.; Boussaad, I.; et al. Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell Rep. 2017, 8, 1144–1154. [Google Scholar] [CrossRef]
- Poli, D.; Magliaro, C.; Ahluwalia, A. Experimental and Computational Methods for the Study of Cerebral Organoids: A Review. Front. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef] [Green Version]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef] [Green Version]
- Amin, N.D.; Pasca, S.P. Building models of brain disorders with three-dimensional organoids. Neuron 2018, 100, 389–405. [Google Scholar] [CrossRef] [Green Version]
- Shaham, S. Glia-neuron interactions in nervous system function and development. Curr. Top. Dev. Biol. 2005, 69, 39–66. [Google Scholar] [CrossRef]
- Qian, X.; Song, H.; Ming, G.L. Brain organoids: Advances, applications and challenges. Development 2019, 146. [Google Scholar] [CrossRef] [Green Version]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Min Yang, S.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Yip, G.M.; Chen, Z.W.; Edge, C.J.; Smith, E.H.; Dickinson, R.; Hohenester, E.; Townsend, R.R.; Fuchs, K.; Sieghart, W.; Evers, A.S.; et al. A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat. Chem. Biol. 2013, 9, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Lancaster, M.A.; Castanon, R.; Nery, J.R.; Knoblich, J.A.; Ecker, J.R. Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell. Rep. 2016, 17, 3369–3384. [Google Scholar] [CrossRef] [Green Version]
- Renner, M.; Lancaster, M.A.; Bian, S.; Choi, H.; Ku, T.; Peer, A.; Chung, K.; Knoblich, J.A. Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 2017, 36, 1316–1329. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, C.A.; Gao, R.; Negraes, P.D.; Gu, J.; Buchanan, J.; Preissl, S.; Wang, A.; Wu, W.; Haddad, G.G.; Chaim, I.A.; et al. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 2019, 25, 558–569. [Google Scholar] [CrossRef]
- Tripathy, S.J.; Toker, L.; Li, B.; Crichlow, C.L.; Tebaykin, D.; Mancarci, B.O.; Pavlidis, P. Transcriptomic correlates of neuron electrophysiological diversity. PLoS Comput. Biol. 2017, 13, e1005814. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.R.; Filipovic, R.; Mo, Z.; Rasband, M.N.; Zecevic, N.; Antic, S.D. Electrical excitability of early neurons in the human cerebral cortex during the second trimester of gestation. Cereb. Cortex 2009, 19, 1795–1805. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.R.; Zhou, W.L.; Jakovcevski, I.; Zecevic, N.; Antic, S.D. Spontaneous electrical activity in the human fetal cortex in vitro. J. Neurosci. 2011, 31, 2391–2398. [Google Scholar] [CrossRef] [Green Version]
- Buss, C.; Entringer, S.; Swanson, J.M.; Wadhwa, P.D. The Role of Stress in Brain Development: The Gestational Environment’s Long-Term Effects on the Brain. Cerebrum 2012, 2012, 4. [Google Scholar]
- Liu, J.; Pasini, S.; Shelanski, M.L.; Greene, L.A. Activating transcription factor 4 (ATF4) modulates post-synaptic development and dendritic spine morphology. Front. Cell. Neurosci. 2014, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Kool, M.J.; Proietti Onori, M.; Borgesius, N.Z.; van de Bree, J.E.; Elgersma-Hooisma, M.; Nio, E.; Bezstarosti, K.; Buitendijk, G.H.S.; Aghadavoud Jolfaei, M.; Demmers, J.A.A.; et al. CAMK2-Dependent Signaling in Neurons Is Essential for Survival. J. Neurosci. 2019, 39, 5424–5439. [Google Scholar] [CrossRef] [Green Version]
- Meganathan, K.; Jagtap, S.; Srinivasan, S.P.; Wagh, V.; Hescheler, J.; Hengstler, J.; Leist, M.; Sachinidis, A. Neuronal developmental gene and miRNA signatures induced by histone deacetylase inhibitors in human embryonic stem cells. Cell Death Dis. 2015, 6, e1756. [Google Scholar] [CrossRef] [Green Version]






| Gene | Forward Sequence 5′ to 3′ | Reverse Sequence 3′ to 5′ | PCR Product Length (bp) |
|---|---|---|---|
| Cd31 | AAGTGGAGTCCAGCCGCATATC | ATGGAGCAGGACAGGTTCAGTC | 133 |
| Gapdh | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA | 131 |
| Map2 | AGGCTGTAGCAGTCCTGAAAGG | CTTCCTCCACTGTGACAGTCTG | 153 |
| S100b | GAAGAAATCCGAACTGAAGGAGC | TCCTGGAAGTCACATTCGCCGT | 135 |
| Scn1a | GGACTGTATGGAGGTTGCTGGT | GCAAGGTTGTCTGCACTAAATGAG | 132 |
| Scn2a | CTAGCCTCACTGTGACAGTACC | TCAACCGTGCTGCCTTCAGATG | 145 |
| Scn3a | CGTCACCTACTGGACAACTTCC | TCACGGCTCTTTGCCTTCCAGA | 129 |
| Scn8a | GGATTGAGACCATGTGGGACTG | ATCTGTGGCAGCCAGGTTGTCT | 158 |
| Scn9a | GTGGAAGGATTGTCAGTTCTGCG | GCCAACACTAAGGTGAGGTTACC | 140 |
| Sma | GATCTGGCACCACTCTTTCTAC | CAGGCAACTCGTAACTCTTCTC | 479 |
| Canonical Pathway [Upregulation, Downregulation, or No Significant Difference (ns) Determined by Z Score Indicated below] | Cerebral Organoids vs. Adult Brains (Z Score) | Fetal Brains vs. Adult Brains (Z Score) | Cerebral Organoid vs. Fetal Brains (Z Score) | Relevance |
|---|---|---|---|---|
| Apoptosis | up (2.196) | ns | ns | Cell death, neurodegeneration |
| Calcium transport | down (−1.633) | ns | ns | Intracellular messaging, cell depolarization, synaptic transmission |
| Dendritic cell maturation | ns | Down (2.019) | ns | Neuron morphology |
| Endocannabinoid developing neuron pathway | down (−2.655) | Down (3.244) | ns | Neuron activity based, differentiation, neuron survival |
| Endocannabinoid neuronal synapse pathway | down (−5.588) | Down (2.142) | ns | Synaptic plasticity, hippocampus signaling |
| Glial derived neurotrophic factor family ligand receptor interactions | down (−2.66) | Down (3.182) | ns | Neurotrophic support, neuron growth, survival |
| Neurotrophin/tyrosine kinase signaling | down (−2.2) | Down (4.382) | ns | Neuron growth and survival |
| Synaptic long-term depression | down (−4.919) | Down (3.354) | ns | Activity-dependent reduction in synaptic signaling |
| Synaptic long-term potentiation | down (−4.608) | Down (2.728) | ns | Activity-dependent strengthening of neuron synapses |
| Calcium signaling | down (−5.661) | down (4.589) | down (−3.250) | Cellular signaling, synaptic transmission |
| CREB signaling neurons | down (−5.578) | down (6.306) | down (−0.911) | Neuron plasticity, neurodevelopment |
| Glutamate receptor signaling | down (−3.3) | Down (2.353) | down (−3.051) | Excitatory neurotransmitter pathway, neurodevelopment |
| Synaptogenesis signaling pathway | down (−7.952) | Down (8.06) | down (−1.741) | Neuron activity based, neuron network formation, pruning, neuron growth factors |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logan, S.; Arzua, T.; Yan, Y.; Jiang, C.; Liu, X.; Yu, L.-K.; Liu, Q.-S.; Bai, X. Dynamic Characterization of Structural, Molecular, and Electrophysiological Phenotypes of Human-Induced Pluripotent Stem Cell-Derived Cerebral Organoids, and Comparison with Fetal and Adult Gene Profiles. Cells 2020, 9, 1301. https://doi.org/10.3390/cells9051301
Logan S, Arzua T, Yan Y, Jiang C, Liu X, Yu L-K, Liu Q-S, Bai X. Dynamic Characterization of Structural, Molecular, and Electrophysiological Phenotypes of Human-Induced Pluripotent Stem Cell-Derived Cerebral Organoids, and Comparison with Fetal and Adult Gene Profiles. Cells. 2020; 9(5):1301. https://doi.org/10.3390/cells9051301
Chicago/Turabian StyleLogan, Sarah, Thiago Arzua, Yasheng Yan, Congshan Jiang, Xiaojie Liu, Lai-Kang Yu, Qing-Song Liu, and Xiaowen Bai. 2020. "Dynamic Characterization of Structural, Molecular, and Electrophysiological Phenotypes of Human-Induced Pluripotent Stem Cell-Derived Cerebral Organoids, and Comparison with Fetal and Adult Gene Profiles" Cells 9, no. 5: 1301. https://doi.org/10.3390/cells9051301
APA StyleLogan, S., Arzua, T., Yan, Y., Jiang, C., Liu, X., Yu, L. -K., Liu, Q. -S., & Bai, X. (2020). Dynamic Characterization of Structural, Molecular, and Electrophysiological Phenotypes of Human-Induced Pluripotent Stem Cell-Derived Cerebral Organoids, and Comparison with Fetal and Adult Gene Profiles. Cells, 9(5), 1301. https://doi.org/10.3390/cells9051301