Iron Speciation of Natural and Anthropogenic Dust by Spectroscopic and Chemical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Aerosol Samples Description
2.3. X-ray Absorption Spectroscopy
2.4. Selective Leaching Experiments
3. Results
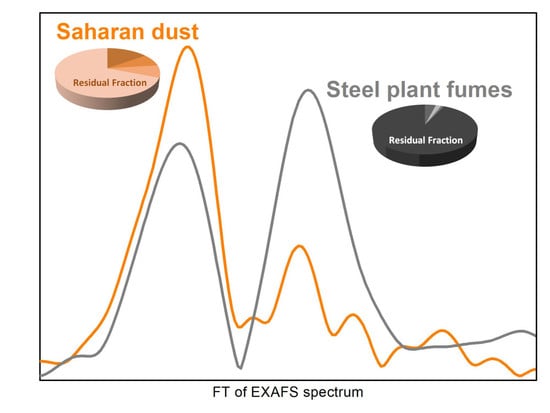
3.1. X-ray Absorption Spectroscopy (XAS) Results
3.2. Leaching Tests Results
4. Discussion
4.1. Saharan Dust
4.2. Steel Production Emissions
4.3. Mixed Urban Dust Cases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| XAS | X-ray Absorption Spectroscopy |
| XANES | X-ray Absorption Near Edge Structure |
| EXAFS | Extended X-ray Absorption Fine Structure |
| ICP-OES | Inductively Coupled Plasma - Optical Emission Spectroscopy |
| EMEP | European Monitoring and Evaluation Program |
| TK-AST | ThyssenKrupp Acciai Speciali Terni |
| MM | Monte Martano |
| WMO SDS-WAS | World Meteorological Organization Sand and Dust Storms—Warning Advisory and Assessment System |
| SDO | Saharan Dust Outbreak |
| BTs | Back Trajectories |
| HVS | High-Volume Sampler |
| ESRF | European Synchrotron Radiation Facility |
| LISA | Linea Italiana di Spettroscopia di Assorbimento a raggi-X |
| CRG | Collaborating Research Group |
References
- Jickells, T.; An, A.; Andersen, K.; Baker, A.; Bergametti, G.; Brooks, N.; Cao, J.; Boyd, P.; Duce, R.; Hunter, K.; et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, Y.; Koren, I.; Rudich, Y.; Artaxo, P.; Martin, S.; Andreae, M. Transport of North African dust from the Bodélé depression to the Amazon Basin: A case study. Atmos. Chem. Phys. 2010, 10, 7533–7544. [Google Scholar] [CrossRef]
- Abouchami, W.; Nathe, K.; Kumar, A.; Galer, S.; Jochum, K.; Williams, E.; Horbe, A.; Rosa, J.; Balsam, W.; Adams, D.; et al. Geochemical and isotopic characterization of the Bodélé Depression dust source and implications for transatlantic dust transport to the Amazonian Basin. Earth Planet. Sci. Lett. 2013, 380, 112–123. [Google Scholar] [CrossRef]
- Smetacek, V.; Klaas, C.; Strass, V.; Assmy, P.; Montresor, M.; Cisewski, B.; Savoye, N.; Webb, A.; d’Ovidio, F.; Arrieta, J.; et al. Deep carbon export from a Southern Ocean iron-fertilized diatom bloom. Nature 2012, 487, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Stafoggia, M.; Zauli-Sajani, S.; Pey, J.; Samoli, E.; Alessandrini, E.; Basagana, X.; Cernigliaro, A.; Chiusolo, M.; Demaria, M.; Dìaz, J.; et al. Desert dust outbreaks in Southern Europe: Contribution to daily PM10 concentrations and short-term associations with mortality and hospital admissions. Environ. Health Perspect. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dall’Osto, M.; Booth, M.; Smith, W.; Fisher, R.; Harrison, R. A study of the size distributions and the chemical characterization of airborne particles in the vicinity of a large integrated steelworks. Aerosol Sci. Technol. 2008, 42, 981–991. [Google Scholar] [CrossRef]
- Hedberg, Y.; Gustafsson, J.; Karlsson, H.; Moller, L.; Wallinder, I. Bioaccessibility, bioavailability and toxicity of commercially relevant iron- and chromium-based particles: In vitro studies with an inhalation perspective. Part. Fibre Toxicol. 2010, 7. [Google Scholar] [CrossRef]
- Smith, K.; Aust, A. Mobilization of iron from urban particulates leads to generation of reactive oxygen species in vitro and induction of ferritin synthesis in human lung epithelial cells. Chem. Res. Toxicol. 1997, 10, 828–834. [Google Scholar] [CrossRef]
- Schroth, A.; Crusius, J.; Sholkovitz, E.; Bostick, B. Iron solubility driven by speciation in dust sources to the ocean. Nat. Geosci. 2009, 2, 337–340. [Google Scholar] [CrossRef]
- Journet, E.; Desboeufs, K.; Caquineau, S.; Colin, J. Mineralogy as a critical factor of dust iron solubility. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.; Jickells, T. Mineral particle size as a control on aerosol iron solubility. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Krom, M.; Jickells, T.; Bonneville, S.; Carslaw, K.; Mihalopoulos, N.; Baker, A.; Benning, L. Impacts on iron solubility in the mineral dust by processes in the source region and the atmosphere: A review. Aeolian Res. 2012, 5, 21–42. [Google Scholar] [CrossRef]
- Mahowald, N.; Baker, A.; Bergametti, G.; Brooks, N.; Duce, R.; Jickells, T.; Kybilay, N.; Prospero, J.; Tegen, I. Atmospheric global dust cycle and iron inputs to the ocean. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef] [Green Version]
- Majestic, B.; Schauer, J.; Shafer, M.; Turner, J.; Fine, P.; Singh, M.; Sioutas, C. Development of a wet-chemical method for the speciation of iron in atmospheric aerosols. Environ. Sci. Technol. 2006, 40, 2346–2351. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Dedik, A.; Ensling, J.; Weinbruch, S.; Weber, S.; Sinner, T.; Gulitch, P.; Ortner, H. Speciation of iron in atmospheric aerosol samples. J. Aerosol Sci. 1996, 27, 325–337. [Google Scholar] [CrossRef]
- Westre, T.; Kennepohl, P.; DeWitt, J.; Hedman, B.; Hodgson, K.; Solomon, E. A multiplet analysis of Fe K-edge 1s-3d pre-edge features of iron complexes. J. Am. Chem. Soc. 1997, 119, 6297–6314. [Google Scholar] [CrossRef]
- Lee, P.; Citrin, P.; Eisenberger, P.; Kincaid, B. Extended X-ray absorption fine structure—Its strenghts and limitations as a structural tool. Rev. Mod. Phys. 1981, 53, 769–806. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, M.; Feng, L.; Li, X. An EXAFS study on the local structure around iron in atmospheric aerosols collected in the Qingdao area. Molecules 2003, 8, 31–39. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Zhang, Y.; Xie, Y.; Li, D.; Zhang, G. Speciation of iron in atmospheric particulate matter by EXAFS. Chin. Sci. Bull. 2006, 51, 2275–2280. [Google Scholar] [CrossRef]
- Elzinga, E.; Gao, Y.; Fitts, J.; Tappero, R. Iron speciation in urban dust. Atmos. Environ. 2011, 45, 4528–4532. [Google Scholar] [CrossRef]
- Oakes, M.; Weber, R.; Lai, B.; Russell, A.; Ingall, E. Characterization of iron speciation in urban and rural single particles using XANES spectroscopy and micro X-ray fluorescence measurements: Investigating the relationships between speciation and fractional iron solubility. Atmos. Chem. Phys. 2012, 12, 745–756. [Google Scholar] [CrossRef]
- d’Acapito, F.; Tagliani, S.M.; Benedetto, F.D.; Gianfagna, A. Local order and valence state of Fe in urban suspended particulate matter. Atmos. Environ. 2014, 99, 582–586. [Google Scholar] [CrossRef]
- Valotto, G.; Cattaruzza, E.; Bardelli, F. Multi-edge X-ray absorption spectroscopy study of road dust samples from a traffic area of Venice using stoichiometric and environmental references. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Marcelli, A.; Hampai, D.; Giannone, F.; Sala, M.; Maggi, V.; Marino, F.; Pignotti, S.; Cibin, G. XRF-XANES characterization of deep ice core insoluble dust. J. Anal. At. Spectrosc. 2012, 27, 33–37. [Google Scholar] [CrossRef]
- Baccolo, G.; Cibin, G.; Delmonte, B.; Hampai, D.; Marcelli, A.; Stefano, E.D.; Macis, S.; Maggi, V. The contribution of synchrotron light for the characterization of atmospheric mineral dust in deep ice cores: Preliminary results from the Talos Dome ice core (East Antarctica). Condens. Matter 2018, 3, 25. [Google Scholar] [CrossRef]
- Lazaro, F.; Gutierrez, L.; Barron, V.; Gelado, M. The speciation of iron in desert dust collected in Gran Canaria (Canary Islands): Combined chemical, magnetic and optical analysis. Atmos. Environ. 2008, 42, 8987–8996. [Google Scholar] [CrossRef]
- Deboudt, K.; Gloter, A.; Mussi, A.; Flament, P. Red-ox speciation and mixing state of iron in individual African dust particles. J. Geophys. Res. 2012, 117. [Google Scholar] [CrossRef] [Green Version]
- Formenti, P.; Caquineau, S.; Chevaillier, S.; Klaver, A.; Desboeufs, K.; Rajot, J.; Belin, S.; Briois, V. Dominance of goethite over hematite in iron oxides of mineral dust from Western Africa: Quantitative partitioning by X-ray absorption spectroscopy. J. Geophys. Res. Atmos. 2014, 119, 12740–12754. [Google Scholar] [CrossRef]
- Nickovic, S.; Vukovic, A.; Vujadinovic, M. Atmospheric processing of iron carried by mineral dust. Atmos. Chem. Phys. 2013, 13, 9169–9181. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Krom, M.; Bonneville, S.; Baker, A.; Bristow, C.; Drake, N.; Mann, G.; Carslaw, K.; McQuaid, J.; Jickells, T.; Benning, L. Influence of chemical weathering and aging of iron oxides on the potential iron solubility of Saharan dust during simulated atmospheric processing. Glob. Biogeochem. Cycles 2011, 25. [Google Scholar] [CrossRef] [Green Version]
- Majestic, B.; Schauer, J.; Shafer, M. Application of synchrotron radiation for measurement of iron red-ox speciation in atmospherically processed aerosols. Atmos. Chem. Phys. 2007, 7, 2475–2487. [Google Scholar] [CrossRef]
- Sammut, M.; Noack, Y.; Rose, J. Zinc speciation in steel plant atmospheric emissions: A multi-technical approach. J. Geochem. Explor. 2006, 88, 239–242. [Google Scholar] [CrossRef]
- Sammut, M.; Noack, Y.; Rose, J.; Hazemann, J.; Proux, O.; Depoux, M.; Ziebel, A.; Fiani, E. Speciation of Cd and Pb in dust emitted from sinter plant. Chemosphere 2010, 78, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Marris, H.; Deboudt, K.; Flament, P.; Grobety, B.; Giere, R. Fe and Mn oxidation states by TEM-EELS in fine-particle emissions from a Fe-Mn alloy making plant. Environ. Sci. Technol. 2013, 47, 10832–10840. [Google Scholar] [CrossRef] [PubMed]
- Moroni, B.; Castellini, S.; Crocchianti, S.; Piazzalunga, A.; Fermo, P.; Scardazza, F.; Cappelletti, D. Ground-based measurements of long-range transported aerosol at the rural regional background site of Monte Martano (Central Italy). Atmos. Res. 2015, 155, 26–36. [Google Scholar] [CrossRef]
- Moroni, B.; Cappelletti, D.; Marmottini, F.; Scardazza, F.; Ferrero, L.; Bolzacchini, E. Integrated single particle-bulk chemical approach for the characterization of local and long range sources of particulate pollutants. Atmos. Environ. 2012, 50, 267–277. [Google Scholar] [CrossRef]
- Federici, E.; Petroselli, C.; Montalbani, E.; Casagrande, C.; Ceci, E.; Moroni, B.; Porta, G.L.; Castellini, S.; Selvaggi, R.; Sebastiani, B.; et al. Airborne bacteria and persistent organic pollutants associated with an intense Saharan dust event in the Central Mediterranean. Sci. Total Environ. 2018, 645, 401–410. [Google Scholar] [CrossRef]
- Petroselli, C.; Crocchianti, S.; Moroni, B.; Castellini, S.; Selvaggi, R.; Nava, S.; Calzolai, G.; Lucarelli, F.; Cappelletti, D. Disentangling the major source areas for an intense aerosol advection in the Central Mediterranean on the basis of Potential Source Contribution Function modeling of chemical and size distribution measurements. Atmos. Res. 2018, 204, 67–77. [Google Scholar] [CrossRef]
- Moroni, B.; Ferrero, L.; Crocchianti, S.; Perrone, S.; Sangiorgi, M.; Bolzacchini, E.; Cappelletti, D. Aerosol dynamics upon Terni basin (Central Italy): Results of integrated vertical profile measurements and electron microscopy analyses. Rendiconti Lincei 2013, 24, 319–328. [Google Scholar] [CrossRef]
- Draxler, R.; Rolph, G. HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) Model Access via NOAA ARL READY. 2012. Available online: http:/ready.arl.noaa.gov/HYSPLIT.php (accessed on 29 December 2018).
- Escudero, M.; Querol, X.; Pey, J.; Alastuey, A.; Perez, N.; Ferreira, F.; Alonso, S.; Rodriguez, S.; Cuevas, E. A methodology for the quantification of the net African dust load in air quality monitoring networks. Atmos. Environ. 2007, 41, 5516–5524. [Google Scholar] [CrossRef]
- d’Acapito, F.; Trapananti, A.; Torrengo, S.; Mobilio, S. X-ray Absorption Spectroscopy: The Italian beamline GILDA of the ESRF. Notiziario Neutroni e Luce di Sincrotrone 2014, 19, 14–23. [Google Scholar]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.; Rauret, G.; Lück, D.; Yli-Halla, M.; Muntau, H.; Quevauviller, P.; Lopez-Sanchez, J. Certification of the extractable contents of Cd, Cr, Cu, Ni, Pb and Zn in a freshwater sediment following a collaboratively tested and optimised three-step sequential extraction procedure. J. Environ. Monit. 2001, 3, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Smichowski, P.; Polla, G.; Gomez, D. Metal fractionation of atmospheric aerosols via sequential chemical extraction: A review. Anal. Bioanal. Chem. 2005, 381, 302–316. [Google Scholar] [CrossRef]
- Wilke, M.; Farges, F.; Petit, P.; Brown, G.; Martin, F. Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- Giuli, G.; Pratesi, G.; Cipriani, C.; Paris, E. Iron local structure in tektites and impact glasses by extended X-ray absorption fine structure and high-resolution X-ray absorption near-edge structure spectroscopy. Geochim. Cosmochim. Acta 2002, 66, 4347–4353. [Google Scholar] [CrossRef]
- Bearden, J.A.; Burr, A.F. Reevaluation of X-ray atomic energy levels. Rev. Mod. Phys. 1967, 39, 125–142. [Google Scholar] [CrossRef]
- O’day, P.; Rivera, N.; Root, R.; Carroll, S. X-ray absorption spectroscopic study of Fe reference compounds for the analysis of natural sediments. Am. Mineral. 2004, 89, 572–585. [Google Scholar] [CrossRef]
- Newville, M. Using Bond Valence sums as restrains in XAFS analysis. Phys. Scr. 2005, T115, 159–161. [Google Scholar] [CrossRef]
- Oliver, S.; Harris, V.; Hamdeh, H.H.; Ho, J.C. Large zinc cation occupancy of octahedral sites in mechanically activated zinc ferrite powders. Appl. Phys. Lett. 2000, 76, 2761–2763. [Google Scholar] [CrossRef]
- Suda, A.; Makino, T.; Higashi, T. Extractability of manganese and iron oxides in typical Japanese soils by 0.5 mol/L hydroxylamine hydrochloride (pH 1.5). Soil Sci. Plant Nutr. 2012, 58, 684–695. [Google Scholar] [CrossRef]
- Suda, A.; Makino, T.; Higashi, T. An improved selective extraction method for Mn oxides and occluded metals with emphasis on applicability to Andisols. Soil Sci. Plant Nutr. 2013, 59, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Dabek-Zlotorzynska, E.; Kelly, M.; Chen, H.; Chakrabarti, C. Evaluation of capillary electrophoresis combined with a BCR sequential extraction for determining distribution of Fe, Zn, Cu, Mn, and Cd in airborne particulate matter. Anal. Chim. Acta 2003, 498, 175–187. [Google Scholar] [CrossRef]
- Coz, E.; Gomez-Moreno, F.; Pujadas, M.; Casuccio, G.S.; Lersch, T.; Artinano, B. Individual particle characteristics of North African dust under different long-range transport scenarios. Atmos. Environ. 2009, 43, 1850–1863. [Google Scholar] [CrossRef]
- Scheuvens, D.; Schutz, L.; Kandler, K.; Ebert, M.; Weinbruch, S. Bulk composition of northern African dust and its source sediments—A compilation. Earth Sci. Rev. 2013, 116, 170–194. [Google Scholar] [CrossRef]
- Hiemstra, T. Surface and mineral structure of ferrihydrite. Geochim. Cosmochim. Acta 2013, 105, 316–325. [Google Scholar] [CrossRef]
- Takahashi, Y.; Higashi, M.; Furukawa, T.; Mitsunobu, S. Change of iron species and iron solubility in Asian dust during the long-range transport from western China to Japan. Atmos. Chem. Phys. 2011, 11, 11237–11252. [Google Scholar] [CrossRef] [Green Version]
- Ebert, M.; Muler-Ebert, D.; Benker, N.; Weinbruch, S. Source apportionment of aerosol particles near a steel plant by electron microscopy. J. Environ. Monit. 2012, 14, 3257–3266. [Google Scholar] [CrossRef]
- Arndt, J.; Deboudt, K.; Anderson, A.; Blondel, A.; Eliet, S.; Flament, P.; Fourmentin, M.; Healy, R.; Savary, V.; Setyan, A.; et al. Scanning electron microscopy-energy dispersive X-ray spectrometry (SEM-EDX) and aerosol time-of-flight mass spectrometry (ATOFMS) single particle analysis of metallurgy plant emissions. Environ. Pollut. 2016, 210, 9–17. [Google Scholar] [CrossRef]
- Moffet, R.; Furutani, H.; Rodel, T.; Henn, T.; Sprau, P.; Laskin, A.; Uematsu, M.; Gilles, M. Iron speciation and mixing in single aerosol particles from the Asian continental outflow. J. Geophys. Res. 2012, 117. [Google Scholar] [CrossRef] [Green Version]
- Anmark, N.; Karasev, A.; Jonsson, P. The effect of different non-metallic inclusions on the machinability of steels. Materials 2015, 8, 751–783. [Google Scholar] [CrossRef] [PubMed]



| Sample | Size Fraction | PM [μg m−3] | Fe [μg m−3] | Fe [%ww] |
|---|---|---|---|---|
| SH_Dec2014 | PM | 86.9 | 1.55 | 1.8% |
| TR_mix1 | PM | 109.9 | 2.30 | 2.1% |
| TR_mix2 | PM | 62.2 | 0.17 | 0.3% |
| AST_E45 | TSP | 1.71 × 10 | 230.4 | 13.5% |
| Sample | Centroid [eV] | Intensity |
|---|---|---|
| SH_Dec2014 | 7114.9 | 0.04 |
| TR_mix1 | 7115.0 | 0.05 |
| TR_mix2 | 7114.5 | 0.09 |
| AST_E45 | 7114.2 | 0.09 |
| Fe-O | Fe-Fe | |||||
|---|---|---|---|---|---|---|
| Sample | r(Fe-O) [Å] | N | [Å] | r(Fe-Fe) [Å] | N | [Å] |
| SH_Dec2014 | 1.99(1) | 6.5(4) | 0.011(3) | 2.98(1) | 1.4(4) | 0.01(1) |
| TR_mix1 | 2.01(1) | 5.0(3) | 0.009(2) | 2.99(1) | 1.8(4) | 0.009(6) |
| TR_mix2 | 2.06(6) | 2.4(1) | 0.02(1) | 2.96(1) | 2.4(1) | 0.02(1) |
| TR_mix2 | 1.94(1) | 2.4(1) | 0.003(1) | 3.48(1) | 7.1(1) | 0.003(1) |
| AST_E45 | 2.06(6) | 3.0(2) | 0.03(2) | 2.98(2) | 3.0(2) | 0.03(2) |
| AST_E45 | 1.93(2) | 1.9(2) | 0.003(5) | 3.50(2) | 5.8(2) | 0.003(5) |
| Sample | I (%) | II (%) | III (%) | IV (%) | tot Fe (μg) |
|---|---|---|---|---|---|
| SH_Dec2014 | 15 | 8 | 8 | 69 | 21.2 |
| AST_E45 | 4 | 2 | 1 | 93 | 308.0 |
| TR_mix1 | 11 | 6 | 5 | 78 | 37.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petroselli, C.; Moroni, B.; Crocchianti, S.; Selvaggi, R.; Vivani, R.; Soggia, F.; Grotti, M.; D’Acapito, F.; Cappelletti, D. Iron Speciation of Natural and Anthropogenic Dust by Spectroscopic and Chemical Methods. Atmosphere 2019, 10, 8. https://doi.org/10.3390/atmos10010008
Petroselli C, Moroni B, Crocchianti S, Selvaggi R, Vivani R, Soggia F, Grotti M, D’Acapito F, Cappelletti D. Iron Speciation of Natural and Anthropogenic Dust by Spectroscopic and Chemical Methods. Atmosphere. 2019; 10(1):8. https://doi.org/10.3390/atmos10010008
Chicago/Turabian StylePetroselli, Chiara, Beatrice Moroni, Stefano Crocchianti, Roberta Selvaggi, Riccardo Vivani, Francesco Soggia, Marco Grotti, Francesco D’Acapito, and David Cappelletti. 2019. "Iron Speciation of Natural and Anthropogenic Dust by Spectroscopic and Chemical Methods" Atmosphere 10, no. 1: 8. https://doi.org/10.3390/atmos10010008
APA StylePetroselli, C., Moroni, B., Crocchianti, S., Selvaggi, R., Vivani, R., Soggia, F., Grotti, M., D’Acapito, F., & Cappelletti, D. (2019). Iron Speciation of Natural and Anthropogenic Dust by Spectroscopic and Chemical Methods. Atmosphere, 10(1), 8. https://doi.org/10.3390/atmos10010008