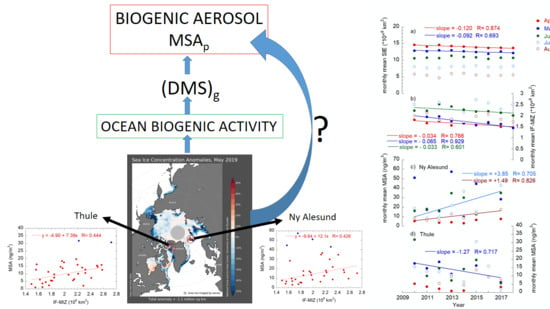
Biogenic Aerosol in the Arctic from Eight Years of MSA Data from Ny Ålesund (Svalbard Islands) and Thule (Greenland)
Abstract
:1. Introduction
2. Methods
2.1. Aerosols Sampling Sites
2.2. Chemical Analysis
2.3. Sea Ice Data
2.3.1. Results
2.3.2. Sea Ice–Biogenic Aerosol Interconnection
2.3.3. North Atlantic Oscillation–Biogenic Aerosol Interconnection
3. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gondwe, M.; Krol, M.; Klaassen, W.; Gieskes, W.; de Baar, H. Comparison of modeled versus measured MSA:nssSO4 ratios: a global analysis. Glob. Biogeochem. Cycles 2004, 18, GB2006. [Google Scholar] [CrossRef]
- Hoffmann, E.H.; Tilgner, A.; Schrödner, R.; Bräuer, P.; Wolke, R.; Herrmann, H. An advanced modeling study on the impacts and atmospheric implications of multiphase dimethyl sulfide chemistry. PNAS 2016, 112, 11776–11781. [Google Scholar] [CrossRef] [PubMed]
- Hodshire, A.L.; Campuzano-Jost, P.; Kodros, J.K.; Croft, B.; Nault, B.A.; Schroder, J.C.; Jimenez, J.L.; Pierce, J.R. The potential role of methanesulfonic acid (MSA) in aerosol formation and growth and the associated radiative forcings. Atmos. Chem. Phys. 2019, 3137–3160. [Google Scholar] [CrossRef]
- Isaksson, E.; Kekonen, T.; Moore, J.; Mulvaney, R. The methanesulfonic acid (MSA) record in a Svalbard ice core. Ann. Glaciol. 2005, 42, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Becagli, S.; Castellano, E.; Cerri, O.; Curran, M.; Frezzotti, M.; Marino, F.; Morganti, A.; Proposito, M.; Severi, M.; Traversi, R.; et al. Methanesulphonic acid (MSA) stratigraphy from a Talos Dome ice core as a tool in depicting sea ice changes and southern atmospheric circulation over the previous 140 years. Atmos. Environ. 2009, 43, 1051–1058. [Google Scholar] [CrossRef]
- Serreze, M.C.; Barry, R.G. Processes and impacts of Arctic amplification: A research synthesis. Glob. Planet. Change 2011, 77, 85–96. [Google Scholar] [CrossRef]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G. Changes in Atmospheric Constituents and in Radiative Forcing. In Climate Change 2007: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Gabric, A.J.; Qu, B.; Matrai, P.; Hirst, A.C. The simulated response of dimethylsulfide production in the Arctic Ocean to global warming. Tellus B 2005, 57, 391–403. [Google Scholar] [CrossRef]
- Boyce, D.G.; Lewis, M.R.; Worm, B. Global phytoplankton decline over the past century. Nature 2010, 466, 591–596. [Google Scholar] [PubMed]
- Bélanger, S.; Babin, M.; Tremblay, J.-E. Increasing cloudiness in Arctic damps the increase in phytoplankton primary production due to sea ice receding. Biogeosciences 2013, 10, 4087–4101. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, K.R.; Perovich, D.K.; Pickart, R.S.; Brown, Z.W.; van Dijken, G.L.; Lowry, K.E.; Mills, M.M.; Palmer, M.A.; Balch, W.M.; Bahr, F.; et al. Massive phytoplankton blooms under Arctic sea ice. Science 2012, 336, 1408. [Google Scholar] [CrossRef] [PubMed]
- Becagli, S.; Ghedini, C.; Peeters, S.; Rottiers, A.; Traversi, R.; Udisti, R.; Chiari, M.; Jalba, A.; Despiau, S.; Dayan, U.; et al. MBAS (methylene blue active substances) and LAS (linear Alkylbenzene sulphonates) in Mediterranean coastal aerosols: sources and transport processes. Atmos. Environ. 2011, 45, 6788–6801. [Google Scholar] [CrossRef]
- Stroeve, J. Sea Ice Trends and Climatologies from SMMR and SSM/I-SSMIS. Sea Ice Extent. NASA DAAC at the National Snow and Ice Data Center: Boulder, CO, USA. Available online: http://nsidc.org/data/nsidc-0192.html (accessed on 19 April 2019).
- Becagli, S.; Lazzara, L.; Marchese, C.; Dayan, U.; Ascanius, S.E.; Cacciani, M.; Caiazzo, L.; Di Biagio, C.; Di Iorio, T.; di Sarra, A.; et al. Relationships linking primary production, sea ice melting, and biogenic aerosol in the Arctic. Atmos. Environ. 2016, 136, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Marchese, C.; Albouy, C.; Tremblay, J.-É.; Dumont, D.; D’Ortenzio, F.; Vissault, S.; Bélanger, S. Changes in phytoplankton bloom phenology over the north water (now) polynya: Aresponse to changing environmental conditions. Polar Biol. 2017, 40, 1721–1737. [Google Scholar] [CrossRef]
- Stirling, I. The importance of polynya, ice edges, and leads to marine mammals and birds. J. Mar. Sys. 1997, 10, 9–21. [Google Scholar] [CrossRef]
- Smith, S.D.; Muench, R.D.; Pease, C.H. Polynyaa and leads: An overview of physical processes and environment. J. Geophys. Res. 1990, 95, 9461–9479. [Google Scholar] [CrossRef]
- Tremblay, J.E.; Gratton, Y.; Fauchota, J.; Price, N.M. Climatic and oceanic forcing of new, net, and diatom production in the North Water. Deep Sea Res. Part II 2002, 49, 4927–4946. [Google Scholar] [CrossRef]
- Guglielmo, L.; Carrada, G.C.; Catalano, G.; Dell’Anno, A.; Fabiano, M.; Lazzara, L.; Mangoni, O.; Pusceddu, A.; Saggiomo, V. Structural and functional properties of sea ice communities in the first year sea ice at Terra Nova Bay (Ross Sea, Antarctica). Polar Biol. 2000, 23, 137–146. [Google Scholar] [CrossRef]
- Lazzara, L.; Nardello, I.; Ermanni, C.; Mangoni, O.; Saggiomo, V. Light environment and seasonal dynamics of microalgae in the annual sea ice at Terra Nova Bay (Ross Sea, Antarctica). Antarct. Sci. 2007, 19, 83–92. [Google Scholar] [CrossRef]
- Levasseur, M. Impact of Arctic meltdown on the microbial cycling of sulphur. Nat. Geogr. 2013, 6, 691–700. [Google Scholar] [CrossRef]
- Lee, P.A.; de Mora, S.J.; Gosselin, M.; Levasseur, M.; Bouillon, R.-C.; Nozais, C.; Michel, C. Particulate dimethylsulfoxide in Arctic sea-ice algal communities: The cryoprotectant hypothesis revisited. J. Phycol. 2001, 37, 488–499. [Google Scholar] [CrossRef]
- Udisti, R.; Bazzano, A.; Becagli, S.; Bolzacchini, E.; Caiazzo, L.; Cappelletti, D.; Ferrero, L.; Frosini, D.; Giardi, F.; Grotti, M.; et al. Sulfate source apportionment in the Ny-Ålesund (Svalbard Islands) Arctic aerosol. Rend. Fis. Acc. Lincei. 2016, 27 (Suppl. S1), 85–94. [Google Scholar] [CrossRef]
- Sharma, S.; Chan, E.; Ishizawa, M.; Toom-Sauntry, D.; Gong, S.L.; Li, S.M.D.; Tarasick, W.; Leaitch, W.R.; Norman, A.; Quinn, P.K.; et al. Influence of transport and ocean ice extent on biogenic aerosol sulfur in the Arctic atmosphere. J. Geophys. Res. 2012, 117, D12209. [Google Scholar] [CrossRef]
- Simó, R.; Pedrós-Alió, C. Role of vertical mixing in controlling the oceanic production of dimethyl sulphide. Nature 1999, 402, 396–399. [Google Scholar] [CrossRef]
- Vallina, S.M.; Simó, R.; Gassó, S. What controls CCN seasonality in the Southern Ocean? A statistical analysis based on satellite-derived chlorophyll and CCN and model-estimated OH radical and rainfall. Global Biogeochem. Cycles 2006, 20, GB1014. [Google Scholar] [CrossRef]
- Sverdrup, H.U. On conditions for the vernal blooming of phytoplankton. J. Cons. int. Explor. 1953, 18, 287–295. [Google Scholar] [CrossRef]
- Henson, S.A.; Dunne, J.P.; Sarmiento, J.L. Decadal variability in North Atlantic phytoplankton blooms. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Mahadevan, A.; D’Asaro, E.; Lee, C.; Perry, M.J. Eddy-driven stratification initiates North Atlantic spring phytoplankton blooms. Science 2012, 337, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Behrenfeld, M. Abandoning Sverdrup’s Critical Depth Hypothesis on phytoplankton blooms. Ecology 2010, 91, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Boss, E.; Behrenfeld, M. In situ evaluation of the initiation of the North Atlantic phytoplankton bloom. Geophys. Res. Lett. 2010, 37, L18603. [Google Scholar] [CrossRef]
- Chiswell, S.M. Annual cycles and spring blooms in phytoplankton: Don’t abandon Sverdrup completely. Mar. Ecol. Prog. Ser. 2011, 443, 39–50. [Google Scholar] [CrossRef]
- Taylor, J.R.; Ferrari, R. Shutdown of turbulent convection as a new criterion for the onset of spring phytoplankton blooms. Limnol. Oceanogr. 2011, 56, 2293–2307. [Google Scholar] [CrossRef] [Green Version]
- Marchese, C.; Castro de la Guardia, L.; Myers, P.G.; Bélanger, S. Regional differences and inter-annual variability in the timing of surface phytoplankton blooms in the Labrador Sea. Ecol. Indic. 2019, 96, 81–90. [Google Scholar] [CrossRef]
- Galí, M.; Simó, R. Occurrence and cycling of dimethylated sulfur compounds in the Arctic during summer receding of the ice edge. Mar. Chem. 2010, 122, 105–117. [Google Scholar] [CrossRef]
- Zhai, L.; Platt, T.; Tang, C.; Sathyendranath, S.; Walne, A. The response of phytoplankton to climate variability associated with the North Atlantic Oscillation. Deep Sea Res. Part II 2013, 93, 159–168. [Google Scholar] [CrossRef]
- Huebert, B.J.; Blomquist, B.W.; Yang, M.X.; Archer, S.D.; Nightingale, P.D.; Yelland, M.J.; Stephens, J.; Pascal, R.W.; Moat, B.I. Linearity of DMS transfer coefficient with both friction velocity and wind speed in the moderate wind speed range. Geophys. Res. Lett. 2010, 37, L01605. [Google Scholar] [CrossRef]
- Park, K.-T.; Lee, K.; Yoon, Y.-J.; Lee, H.-W.; Kim, H.-C.; Lee, B.-Y.; Hermansen, O.; Kim, T.-W.; Holmén, K. Linking atmospheric dimethyl sulfide and the Arctic Ocean spring bloom. Geophys. Res. Lett. 2013, 40, 155–160. [Google Scholar] [CrossRef]





| Thule S-BioAer | Ny Ålesund S-BioAer | |||
|---|---|---|---|---|
| ng/m3 | % of PM10 | ng/m3 | % of PM10 | |
| (nssSO42−/MSA)bio = 3 Mean ± Std Dev. Apr–Sep | 41.3 ± 36.5 | 1.8 ± 1.6% | 72.8 ± 92.7 | 2.7 ± 3.5% |
| (nssSO42−/MSA)bio = 6 Mean ± Std Dev. Apr–Sep | 72.4 ± 63.8 | 3.1 ± 2.8% | 127.4 ± 162.3 | 4.8 ± 6.1% |
| (nssSO42−/MSA)bio = 3 Mean ± Std Dev May–Jul | 57.2 ± 39.4 | 2.6 ± 1.8% | 105.4 ± 109.0 | 4.1 ± 4.2% |
| (nssSO42−/MSA)bio = 6 Mean ± Std Dev May–Jul | 100.1 ± 69.0 | 4.5 ± 3.1% | 184.5 ± 190.7 | 7.1 ± 7.4% |
| MSA Thule | MSA Ny Ålesund | |||||||
|---|---|---|---|---|---|---|---|---|
| Slope | R | n. | p | Slope | R | n. | p | |
| SIA (AMJ) | −1.94 | 0.548 | 20 | <0.05 | −4.18 | 0.720 | 18 | <0.01 |
| SIE (AMJ) | −2.30 | 0.550 | 20 | <0.05 | −4.98 | 0.722 | 18 | <0.01 |
| SIA (JA) | +0.732 | 0.180 | 13 | - | +6.69 | 0.628 | 13 | <0.05 |
| SIE (JA) | +0.948 | 0.285 | 13 | - | +4.87 | 0.557 | 13 | <0.05 |
| IF-MIZ | +7.39 | 0.444 | 33 | <0.01 | +16.3 | 0.426 | 31 | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becagli, S.; Amore, A.; Caiazzo, L.; Iorio, T.D.; Sarra, A.d.; Lazzara, L.; Marchese, C.; Meloni, D.; Mori, G.; Muscari, G.; et al. Biogenic Aerosol in the Arctic from Eight Years of MSA Data from Ny Ålesund (Svalbard Islands) and Thule (Greenland). Atmosphere 2019, 10, 349. https://doi.org/10.3390/atmos10070349
Becagli S, Amore A, Caiazzo L, Iorio TD, Sarra Ad, Lazzara L, Marchese C, Meloni D, Mori G, Muscari G, et al. Biogenic Aerosol in the Arctic from Eight Years of MSA Data from Ny Ålesund (Svalbard Islands) and Thule (Greenland). Atmosphere. 2019; 10(7):349. https://doi.org/10.3390/atmos10070349
Chicago/Turabian StyleBecagli, Silvia, Alessandra Amore, Laura Caiazzo, Tatiana Di Iorio, Alcide di Sarra, Luigi Lazzara, Christian Marchese, Daniela Meloni, Giovanna Mori, Giovanni Muscari, and et al. 2019. "Biogenic Aerosol in the Arctic from Eight Years of MSA Data from Ny Ålesund (Svalbard Islands) and Thule (Greenland)" Atmosphere 10, no. 7: 349. https://doi.org/10.3390/atmos10070349
APA StyleBecagli, S., Amore, A., Caiazzo, L., Iorio, T. D., Sarra, A. d., Lazzara, L., Marchese, C., Meloni, D., Mori, G., Muscari, G., Nuccio, C., Pace, G., Severi, M., & Traversi, R. (2019). Biogenic Aerosol in the Arctic from Eight Years of MSA Data from Ny Ålesund (Svalbard Islands) and Thule (Greenland). Atmosphere, 10(7), 349. https://doi.org/10.3390/atmos10070349