A Review of Ongoing Advancements in Soil and Water Assessment Tool (SWAT) for Nitrous Oxide (N2o) Modeling
Abstract
:1. Why Is N2O Important?
2. Sources and Sinks of N2O
3. N2O Controlling Factors
3.1. Environmental Factors
3.1.1. Weather
3.1.2. Freeze–Thaw Cycles
3.2. Land- and Crop-Management Factors
3.2.1. Tillage
3.2.2. Fertilizers
3.2.3. Residues
3.2.4. Cover Crops
3.3. Soil Characteristics
3.3.1. Soil Type
3.3.2. Soil Temperature
3.3.3. Water-Filled Pore Spaces
3.3.4. Compaction
3.3.5. Carbon and Nitrogen
3.3.6. The pH
4. Capability of Process-Based Models for N2O Emission Modeling
5. Introduction to SWAT
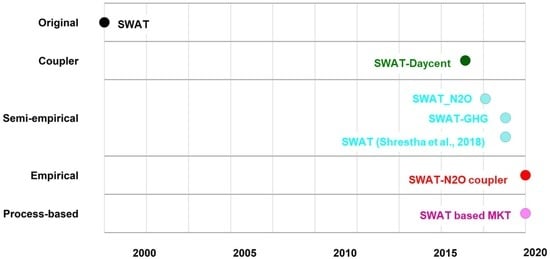
6. Advancements of SWAT in Simulating N2O Emissions
6.1. Coupler Revisions
6.2. Semi-Empirical Revisions
6.3. Empirical Revisions
6.4. Process-Based Revisions
7. Limitations
8. Recommendation
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.; Kutz, S.; Harvell, C.D. Climate change and infectious diseases: From evidence to a predictive framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.A. Nitrous Oxide and Climate Change; Earthscan: London, UK, 2010. [Google Scholar]
- Neubauer, S.C.; Megonigal, J.P. Moving beyond global warming potentials to quantify the climatic role of ecosystems. Ecosystems 2015, 18, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, O.; Stone, K.A.; Schofield, R.; Akiyoshi, H.; Yamashita, Y.; Kinnison, D.E.; Garcia, R.R.; Sudo, K.; Plummer, D.A.; Scinocca, J. Ozone sensitivity to varying greenhouse gases and ozone-depleting substances in CCMI-1 simulations. Atmos. Chem. Phys. 2018, 18, 1091–1114. [Google Scholar] [CrossRef] [Green Version]
- Lehnert, N.; Dong, H.T.; Harland, J.B.; Hunt, A.P.; White, C.J. Reversing nitrogen fixation. Nat. Rev. Chem. 2018, 2, 278–289. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef]
- Remigi, P.; Zhu, J.; Young, J.P.W.; Masson-Boivin, C. Symbiosis within symbiosis: Evolving nitrogen-fixing legume symbionts. Trends Microbiol. 2016, 24, 63–75. [Google Scholar] [CrossRef]
- Wagner, S. Biological Nitrogen Fixation. Nat. Educ. Knowl. 2011, 3, 15. [Google Scholar]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636. [Google Scholar] [CrossRef]
- Bernhard, A.; The Nitrogen Cycle: Processes, Players, and Human IMPACT [WWW Document]. Nature Education Knowledge 2010. Available online: https://www.nature.com/scitable/knowledge/library/the-nitrogen-cycle-processes-players-and-human-15644632/ (accessed on 2 April 2020).
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Norton, J.M. Nitrification in agricultural soils. Agronomy 2008, 49, 173. [Google Scholar]
- Blackmer, A.; Bremner, J.; Schmidt, E. Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil. Appl. Environ. Microbiol. 1980, 40, 1060–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, T.; Stratton, G.; Gordon, R.; Madani, A. The use of aeration to enhance ammonia nitrogen removal in constructed wetlands. Can. Biosyst. Eng. 2003, 45, 1–9. [Google Scholar]
- Grundmann, G.; Renault, P.; Rosso, L.; Bardin, R. Differential effects of soil water content and temperature on nitrification and aeration. Soil Sci. Soc. Am. J. 1995, 59, 1342–1349. [Google Scholar] [CrossRef]
- Gilmour, J. The effects of soil properties on nitrification and nitrification inhibition. Soil Sci. Soc. Am. J. 1984, 48, 1262–1266. [Google Scholar] [CrossRef]
- Ward, B.B.; Arp, D.J.; Klotz, M.G. Nitrification; American Society for Microbiology Press: Washington, DC, USA, 2011. [Google Scholar]
- Capone, D.G.; Bronk, D.A.; Mulholland, M.R.; Carpenter, E.J. Nitrogen in the Marine Environment; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Wunderlin, P.; Mohn, J.; Joss, A.; Emmenegger, L.; Siegrist, H. Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res. 2012, 46, 1027–1037. [Google Scholar] [CrossRef]
- Verstraete, W.; Focht, D. Biochemical ecology of nitrification and denitrification. In Advances in Microbial Ecology; Springer: Berlin/Heidelberg, Germany, 1977; pp. 135–214. [Google Scholar]
- Gamble, T.N.; Betlach, M.R.; Tiedje, J.M. Numerically dominant denitrifying bacteria from world soils. Appl. Environ. Microbiol. 1977, 33, 926–939. [Google Scholar] [CrossRef] [Green Version]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Bateman, E.; Baggs, E. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Li, X.; Sørensen, P.; Olesen, J.E.; Petersen, S.O. Evidence for denitrification as main source of N2O emission from residue-amended soil. Soil Biol. Biochem. 2016, 92, 153–160. [Google Scholar] [CrossRef]
- Morse, J.L.; Bernhardt, E.S. Using 15N tracers to estimate N2O and N2 emissions from nitrification and denitrification in coastal plain wetlands under contrasting land-uses. Soil Biol. Biochem. 2013, 57, 635–643. [Google Scholar] [CrossRef]
- Schlesinger, W.H. On the fate of anthropogenic nitrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakken, L.R.; Frostegård, Å. Sources and sinks for N2O, can microbiologist help to mitigate N2O emissions. Environ. Microbiol. 2017, 19, 4801–4805. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, N.; Coruzzi, G.; Hegg, E.; Seefeldt, L.; Stein, L. NSF Workshop Report: Feeding the World in the 21st Century: Grand Challenges in the Nitrogen Cycle. 2017. Available online: http://umich.edu/~lehnert/Ncycle.html (accessed on 2 April 2020).
- Wrage, N.; Lauf, J.; del Prado, A.; Pinto, M.; Pietrzak, S.; Yamulki, S.; Oenema, O.; Gebauer, G. Distinguishing sources of N2O in European grasslands by stable isotope analysis. Rapid Commun. Mass Spectrom. 2004, 18, 1201–1207. [Google Scholar] [CrossRef]
- Ussiri, D.; Lal, R. Global sources of nitrous oxide. In Soil Emission of Nitrous Oxide and Its Mitigation; Springer: Berlin/Heidelberg, Germany, 2013; pp. 131–175. [Google Scholar]
- Laville, P.; Lehuger, S.; Loubet, B.; Chaumartin, F.; Cellier, P. Effect of management, climate and soil conditions on N2O and NO emissions from an arable crop rotation using high temporal resolution measurements. Agric. For. Meteorol. 2011, 151, 228–240. [Google Scholar] [CrossRef]
- Paudel, S.R.; Choi, O.; Khanal, S.K.; Chandran, K.; Kim, S.; Lee, J.W. Effects of temperature on nitrous oxide (N2O) emission from intensive aquaculture system. Sci. Total Environ. 2015, 518, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Iqbal, J.; Hu, R.; Feng, M. N2O emissions from different land uses in mid-subtropical China. Agric. Ecosyst. Environ. 2010, 136, 40–48. [Google Scholar] [CrossRef]
- Del Grosso, S.; Ogle, S.; Parton, W.; Breidt, F. Estimating uncertainty in N2O emissions from US cropland soils. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, G.; Brouder, S.M.; Smith, D.R.; Van Scoyoc, G.E. Greenhouse gas fluxes in an eastern corn belt soil: Weather, nitrogen source, and rotation. J. Environ. Qual. 2009, 38, 841–854. [Google Scholar] [CrossRef] [Green Version]
- Fuß, R.; Ruth, B.; Schilling, R.; Scherb, H.; Munch, J.C. Pulse emissions of N2O and CO2 from an arable field depending on fertilization and tillage practice. Agric. Ecosyst. Environ. 2011, 144, 61–68. [Google Scholar] [CrossRef]
- Rashti, M.R.; Wang, W.; Moody, P.; Chen, C.; Ghadiri, H. Fertiliser-induced nitrous oxide emissions from vegetable production in the world and the regulating factors: A review. Atmos. Environ. 2015, 112, 225–233. [Google Scholar] [CrossRef]
- Chen, S.; Ouyang, W.; Hao, F.; Zhao, X. Combined impacts of freeze–thaw processes on paddy land and dry land in Northeast China. Sci. Total Environ. 2013, 456, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.A. Soil freeze–thaw cycle experiments: Trends, methodological weaknesses and suggested improvements. Soil Biol. Biochem. 2007, 39, 977–986. [Google Scholar] [CrossRef]
- Risk, N.; Snider, D.; Wagner-Riddle, C. Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze–thaw cycles. Can. J. Soil Sci. 2013, 93, 401–414. [Google Scholar] [CrossRef]
- Wagner-Riddle, C.; Congreves, K.A.; Abalos, D.; Berg, A.A.; Brown, S.E.; Ambadan, J.T.; Gao, X.; Tenuta, M. Globally important nitrous oxide emissions from croplands induced by freeze–thaw cycles. Nat. Geosci. 2017, 10, 279–283. [Google Scholar] [CrossRef]
- Dobbie, K.; Smith, K. The effects of temperature, water-filled pore space and land use on N2O emissions from an imperfectly drained gleysol. Eur. J. Soil Sci. 2001, 52, 667–673. [Google Scholar] [CrossRef]
- Schaufler, G.; Kitzler, B.; Schindlbacher, A.; Skiba, U.; Sutton, M.; Zechmeister-Boltenstern, S. Greenhouse gas emissions from European soils under different land use: Effects of soil moisture and temperature. Eur. J. Soil Sci. 2010, 61, 683–696. [Google Scholar] [CrossRef]
- Vilain, G.; Garnier, J.; Tallec, G.; Cellier, P. Effect of slope position and land use on nitrous oxide (N2O) emissions (Seine Basin, France). Agric. For. Meteorol. 2010, 150, 1192–1202. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Zheng, X.; Ren, X.; Cardenas, L.; Carswell, A.; Misselbrook, T. Land-use type affects N2O production pathways in subtropical acidic soils. Environ. Pollut. 2018, 237, 237–243. [Google Scholar] [CrossRef]
- Katulanda, P.M. Land Use Legacy Regulates Microbial Community Structure And Function In Transplanted Chernozems. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Cananda, 2018. [Google Scholar]
- Shrestha, N.K.; Thomas, B.W.; Du, X.; Hao, X.; Wang, J. Modeling nitrous oxide emissions from rough fescue grassland soils subjected to long-term grazing of different intensities using the Soil and Water Assessment Tool (SWAT). Environ. Sci. Pollut. Res. 2018, 25, 27362–27377. [Google Scholar] [CrossRef]
- Wang, J.; Cardenas, L.M.; Misselbrook, T.H.; Cuttle, S.; Thorman, R.E.; Li, C. Modelling nitrous oxide emissions from grazed grassland systems. Environ. Pollut. 2012, 162, 223–233. [Google Scholar] [CrossRef]
- Mei, K.; Wang, Z.; Huang, H.; Zhang, C.; Shang, X.; Dahlgren, R.A.; Zhang, M.; Xia, F. Stimulation of N2O emission by conservation tillage management in agricultural lands: A meta-analysis. Soil Tillage Res. 2018, 182, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Horak, J.; Igaz, D.; Kondrlova, E.; Cimo, J.; Zembery, J.; Candrakova, E. Effect of conventional tillage and reduced tillage on N2O emission from a loamy soil under spring barley. Int. Multidiscip. Sci. GeoConference: SGEM: Surv. Geol. Min. Ecol. Manag. [CrossRef]
- Venterea, R.T.; Maharjan, B.; Dolan, M.S. Fertilizer source and tillage effects on yield-scaled nitrous oxide emissions in a corn cropping system. J. Environ. Qual. 2011, 40, 1521–1531. [Google Scholar] [CrossRef] [Green Version]
- Kessel, C.v.; Venterea, R.; Six, J.; Adviento-Borbe, M.A.; Linquist, B.; Groenigen, K.J.v. Climate, duration, and N placement determine N2O emissions in reduced tillage systems: A meta-analysis. Glob. Chang. Biol. 2013, 19, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Wanyama, I.; Rufino, M.C.; Pelster, D.E.; Wanyama, G.; Atzberger, C.; Van Asten, P.; Verchot, L.V.; Butterbach-Bahl, K. Land use, land use history, and soil type affect soil greenhouse gas fluxes from agricultural landscapes of the East African Highlands. J. Geophys. Res.: Biogeosci. 2018, 123, 976–990. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [Green Version]
- Millar, N.; Robertson, G.P.; Grace, P.R.; Gehl, R.J.; Hoben, J.P. Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (Maize) production: An emissions reduction protocol for US Midwest agriculture. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Akram, R.; Turan, V.; Wahid, A.; Ijaz, M.; Shahid, M.A.; Kaleem, S.; Hafeez, A.; Maqbool, M.M.; Chaudhary, H.J.; Munis, M.F.H. Paddy land pollutants and their role in climate change. In Environmental Pollution of Paddy Soils; Springer: Berlin/Heidelberg, Germany, 2018; pp. 113–124. [Google Scholar]
- Wang, F.; Li, J.; Wang, X.; Zhang, W.; Zou, B.; Neher, D.A.; Li, Z. Nitrogen and phosphorus addition impact soil N2O emission in a secondary tropical forest of South China. Sci. Rep. 2014, 4, 5615. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.; Famulari, D.; Di Marco, C.; Nemitz, E.; Skiba, U.; Rees, R.; Sutton, M. Nitrous oxide emissions from managed grassland: A comparison of eddy covariance and static chamber measurements. Atmos. Meas. Tech. 2011, 4, 2179–2194. [Google Scholar] [CrossRef] [Green Version]
- Melaku, N.D.; Shrestha, N.K.; Wang, J.; Thorman, R.E. Predicting nitrous oxide emissions following the application of solid manure to grassland in the United Kingdom. J. Environ. Qual. 2020. [Google Scholar] [CrossRef]
- Pathak, H.; Nedwell, D. Nitrous oxide emission from soil with different fertilizers, water levels and nitrification inhibitors. Water Air Soil Pollut. 2001, 129, 217–228. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Snyder, C.S.; Blaylock, A.D.; Del Grosso, S.J. Enhanced-efficiency nitrogen fertilizers: Potential role in nitrous oxide emission mitigation. Agron. J. 2014, 106, 715–722. [Google Scholar] [CrossRef]
- Linquist, B.A.; Liu, L.; van Kessel, C.; van Groenigen, K.J. Enhanced efficiency nitrogen fertilizers for rice systems: Meta-analysis of yield and nitrogen uptake. Field Crops Res. 2013, 154, 246–254. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Wang, J. Current and future hot-spots and hot-moments of nitrous oxide emission in a cold climate river basin. Environ. Pollut. 2018, 239, 648–660. [Google Scholar] [CrossRef]
- Ding, W.; Luo, J.; Li, J.; Yu, H.; Fan, J.; Liu, D. Effect of long-term compost and inorganic fertilizer application on background N2O and fertilizer-induced N2O emissions from an intensively cultivated soil. Sci. Total Environ. 2013, 465, 115–124. [Google Scholar] [CrossRef]
- Pimentel, L.G.; Weiler, D.A.; Pedroso, G.M.; Bayer, C. Soil N2O emissions following cover-crop residues application under two soil moisture conditions. J. Plant Nutr. Soil Sci. 2015, 178, 631–640. [Google Scholar] [CrossRef]
- Jia, J.; Li, B.; Chen, Z.; Xie, Z.; Xiong, Z. Effects of biochar application on vegetable production and emissions of N2O and CH4. Soil Sci. Plant Nutr. 2012, 58, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.; Baligar, V.; Bailey, B. Role of cover crops in improving soil and row crop productivity. Commun. Soil Sci. Plant Anal. 2005, 36, 2733–2757. [Google Scholar] [CrossRef]
- Basche, A.D.; Miguez, F.E.; Kaspar, T.C.; Castellano, M.J. Do cover crops increase or decrease nitrous oxide emissions? A meta-analysis. J. Soil Water Conserv. 2014, 69, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.C.; Castellano, M.J.; Sawyer, J.E.; Pantoja, J. Cover crop effects on nitrous oxide emissions: Role of mineralizable carbon. Soil Sci. Soc. Am. J. 2013, 77, 1765–1773. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.-Q.; Guang-Xi, X.; Zhao-Liang, Z. Nitrous oxide and methane emissions as affected by water, soil and nitrogen. Pedosphere 2007, 17, 146–155. [Google Scholar] [CrossRef]
- Harrison-Kirk, T.; Beare, M.; Meenken, E.; Condron, L. Soil organic matter and texture affect responses to dry/wet cycles: Effects on carbon dioxide and nitrous oxide emissions. Soil Biol. Biochem. 2013, 57, 43–55. [Google Scholar] [CrossRef]
- Skiba, U.; Ball, B. The effect of soil texture and soil drainage on emissions of nitric oxide and nitrous oxide. Soil Use Manag. 2002, 18, 56–60. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Butterbach-Bahl, K. Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J. Geophys. Res.: Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Marhan, S.; Auber, J.; Poll, C. Additive effects of earthworms, nitrogen-rich litter and elevated soil temperature on N2O emission and nitrate leaching from an arable soil. Appl. Soil Ecol. 2015, 86, 55–61. [Google Scholar] [CrossRef]
- Lai, T.V.; Farquharson, R.; Denton, M.D. High soil temperatures alter the rates of nitrification, denitrification and associated N2O emissions. J. Soils Sediments 2019, 19, 2176–2189. [Google Scholar] [CrossRef]
- Horváth, L.; Grosz, B.; Machon, A.; Tuba, Z.; Nagy, Z.; Czóbel, S.; Balogh, J.; Péli, E.; Fóti, S.; Weidinger, T. Estimation of nitrous oxide emission from Hungarian semi-arid sandy and loess grasslands; effect of soil parameters, grazing, irrigation and use of fertilizer. Agric. Ecosyst. Environ. 2010, 139, 255–263. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Banerjee, S.; Helgason, B.; Wang, L.; Winsley, T.; Ferrari, B.C.; Siciliano, S.D. Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol. Biochem. 2016, 95, 40–50. [Google Scholar] [CrossRef]
- Dalal, R.C.; Wang, W.; Robertson, G.P.; Parton, W.J. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review. Soil Res. 2003, 41, 165–195. [Google Scholar] [CrossRef]
- Bessou, C.; Mary, B.; Léonard, J.; Roussel, M.; Gréhan, E.; Gabrielle, B. Modelling soil compaction impacts on nitrous oxide emissions in arable fields. Eur. J. Soil Sci. 2010, 61, 348–363. [Google Scholar] [CrossRef]
- Tullberg, J.; Antille, D.L.; Bluett, C.; Eberhard, J.; Scheer, C. Controlled traffic farming effects on soil emissions of nitrous oxide and methane. Soil Tillage Res. 2018, 176, 18–25. [Google Scholar] [CrossRef]
- Ball, B.; Cameron, K.; Di, H.; Moore, S. Effects of trampling of a wet dairy pasture soil on soil porosity and on mitigation of nitrous oxide emissions by a nitrification inhibitor, dicyandiamide. Soil Use Manag. 2012, 28, 194–201. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.; Zhang, Y.; Lin, Z.; Zhu, T.; Sun, R.; Wang, X.; Ma, J.; Bei, Q.; Liu, G. Can biochar alleviate soil compaction stress on wheat growth and mitigate soil N2O emissions? Soil Biol. Biochem. 2017, 104, 8–17. [Google Scholar] [CrossRef]
- Ma, B.; Wu, T.; Tremblay, N.; Deen, W.; Morrison, M.; McLaughlin, N.; Gregorich, E.; Stewart, G. Nitrous oxide fluxes from corn fields: On-farm assessment of the amount and timing of nitrogen fertilizer. Glob. Chang. Biol. 2010, 16, 156–170. [Google Scholar] [CrossRef]
- Allen, D.; Kingston, G.; Rennenberg, H.; Dalal, R.; Schmidt, S. Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agric. Ecosyst. Environ. 2010, 136, 209–217. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different C: N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Richardson, D.; Felgate, H.; Watmough, N.; Thomson, A.; Baggs, E. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle–could enzymic regulation hold the key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef]
- Liu, B.; Mørkved, P.T.; Frostegård, Å.; Bakken, L.R. Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol. Ecol. 2010, 72, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Wagena, M.B.; Bock, E.M.; Sommerlot, A.R.; Fuka, D.R.; Easton, Z.M. Development of a nitrous oxide routine for the SWAT model to assess greenhouse gas emissions from agroecosystems. Environ. Model. Softw. 2017, 89, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, M. Nitrogen modeling for soil management. J. Soil Water Conserv. 2002, 57, 417–425. [Google Scholar]
- Li, C.; Frolking, S.; Frolking, T.A. A model of nitrous oxide evolution from soil driven by rainfall events: 1. Model structure and sensitivity. J. Geophys. Res.: Atmos. 1992, 97, 9759–9776. [Google Scholar] [CrossRef]
- Parton, W. The CENTURY model. In Evaluation of Soil Organic Matter Models; Springer: Berlin/Heidelberg, Germany, 1996; pp. 283–291. [Google Scholar]
- Parton, W.J.; Hartman, M.; Ojima, D.; Schimel, D. DAYCENT and its land surface submodel: Description and testing. Glob. Planet. Chang. 1998, 19, 35–48. [Google Scholar] [CrossRef]
- Xu, C.; Shaffer, M.; Al-Kaisi, M. Simulating the impact of management practices on nitrous oxide emissions. Soil Sci. Soc. Am. J. 1998, 62, 736–742. [Google Scholar] [CrossRef]
- Kaharabata, S.; Drury, C.; Priesack, E.; Desjardins, R.; McKenney, D.; Tan, C.; Reynolds, D. Comparing measured and Expert-N predicted N2O emissions from conventional till and no till corn treatments. Nutr. Cycl. Agroecosystems 2003, 66, 107–118. [Google Scholar] [CrossRef]
- Grant, R.; Pattey, E. Mathematical modeling of nitrous oxide emissions from an agricultural field during spring thaw. Glob. Biogeochem. Cycles 1999, 13, 679–694. [Google Scholar] [CrossRef]
- Ahuja, L.; Rojas, K.; Hanson, J. Root Zone Water Quality Model: Modelling Management Effects on Water Quality and Crop Production; LLC, P.O. Box 260026, Highlands Ranch, Colorado 80163-0026, U.S.A; Water Resources Publication: Washington, DC, USA, 2000. [Google Scholar]
- Congreves, K.; Wagner-Riddle, C.; Si, B.; Clough, T. Nitrous oxide emissions and biogeochemical responses to soil freezing-thawing and drying-wetting. Soil Biol. Biochem. 2018, 117, 5–15. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Grace, P.; Mosier, A.R. N2O emissions from agricultural lands: A synthesis of simulation approaches. Plant Soil 2008, 309, 169–189. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Zheng, X.; Zhou, Z.; Cui, F.; Zhu, B.; Haas, E.; Klatt, S.; Butterbach-Bahl, K.; Kiese, R. Comparison of the DNDC, LandscapeDNDC and IAP-N-GAS models for simulating nitrous oxide and nitric oxide emissions from the winter wheat–summer maize rotation system. Agric. Syst. 2015, 140, 1–10. [Google Scholar] [CrossRef]
- Regaert, D.; Aubinet, M.; Moureaux, C. Mitigating N2O emissions from agriculture: A review of the current knowledge on soil system modelling, environmental factors and management practices influencing emissions. J. Soil Sci. Environ. Manag. 2015, 6, 178–186. [Google Scholar]
- Gaillard, R.K.; Jones, C.D.; Ingraham, P.; Collier, S.; Izaurralde, R.C.; Jokela, W.; Osterholz, W.; Salas, W.; Vadas, P.; Ruark, M.D. Underestimation of N2O emissions in a comparison of the DayCent, DNDC, and EPIC models. Ecol. Appl. 2018, 28, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, F.; Soussana, J.F.; Bellocchi, G.; Grace, P.; McAuliffe, R.; Recous, S.; Sándor, R.; Smith, P.; Snow, V.; de Antoni Migliorati, M. Assessing uncertainties in crop and pasture ensemble model simulations of productivity and N2O emissions. Glob. Chang. Biol. 2018, 24, e603–e616. [Google Scholar] [CrossRef] [Green Version]
- Groffman, P.M.; Butterbach-Bahl, K.; Fulweiler, R.W.; Gold, A.J.; Morse, J.L.; Stander, E.K.; Tague, C.; Tonitto, C.; Vidon, P. Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 2009, 93, 49–77. [Google Scholar] [CrossRef]
- Arnold, J.G.; Moriasi, D.N.; Gassman, P.W.; Abbaspour, K.C.; White, M.J.; Srinivasan, R.; Santhi, C.; Harmel, R.; Van Griensven, A.; Van Liew, M.W. SWAT: Model use, calibration, and validation. Trans. ASABE 2012, 55, 1491–1508. [Google Scholar] [CrossRef]
- Arnold, J.G.; Srinivasan, R.; Muttiah, R.S.; Williams, J.R. Large area hydrologic modeling and assessment part I: Model development 1. JAWRA J. Am. Water Resour. Assoc. 1998, 34, 73–89. [Google Scholar] [CrossRef]
- Krysanova, V.; Arnold, J.G. Advances in ecohydrological modelling with SWAT—A review. Hydrol. Sci. J. 2008, 53, 939–947. [Google Scholar] [CrossRef]
- Francesconi, W.; Srinivasan, R.; Pérez-Miñana, E.; Willcock, S.P.; Quintero, M. Using the Soil and Water Assessment Tool (SWAT) to model ecosystem services: A systematic review. J. Hydrol. 2016, 535, 625–636. [Google Scholar] [CrossRef]
- Williams, J.; Arnold, J.; Kiniry, J.; Gassman, P.; Green, C. History of model development at Temple, Texas. Hydrol. Sci. J. 2008, 53, 948–960. [Google Scholar] [CrossRef] [Green Version]
- Arnold, J.G.; Gassman, P.W.; White, M.J. New developments in the SWAT ecohydrology model. In Proceedings of the 21st Century Watershed Technology: Improving Water Quality and Environment Conference Proceedings, Universidad EARTH, Limón, San José, Mercedes, Costa Rica, 21–24 February 2010; p. 1. [Google Scholar]
- Tan, M.L.; Gassman, P.W.; Srinivasan, R.; Arnold, J.G.; Yang, X. A review of swat studies in southeast asia: Applications, challenges and future directions. Water 2019, 11, 914. [Google Scholar] [CrossRef] [Green Version]
- Baffaut, C.; Sadeghi, A. Bacteria modeling with SWAT for assessment and remediation studies: A review. Trans. ASABE 2010, 53, 1585–1594. [Google Scholar] [CrossRef]
- Krysanova, V.; White, M. Advances in water resources assessment with SWAT—an overview. Hydrol. Sci. J. 2015, 60, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Liu, S.; Qiu, L.; Sun, Y. SWAT-DayCent coupler: An integration tool for simultaneous hydro-biogeochemical modeling using SWAT and DayCent. Environ. Model. Softw. 2016, 86, 81–90. [Google Scholar] [CrossRef]
- Jayakrishnan, R.; Srinivasan, R.; Santhi, C.; Arnold, J. Advances in the application of the SWAT model for water resources management. Hydrol. Process.: Int. J. 2005, 19, 749–762. [Google Scholar] [CrossRef]
- Gramig, B.M.; Reeling, C.J.; Cibin, R.; Chaubey, I. Environmental and economic trade-offs in a watershed when using corn stover for bioenergy. Environ. Sci. Technol. 2013, 47, 1784–1791. [Google Scholar] [CrossRef]
- Reeling, C.J.; Gramig, B.M. A novel framework for analysis of cross-media environmental effects from agricultural conservation practices. Agric. Ecosyst. Environ. 2012, 146, 44–51. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, Y.; Sivakumar, B.; Long, A.; Qiu, L.; Chen, J.; Wang, L.; Liu, S.; Hu, H. Climatic and hydrologic controls on net primary production in a semiarid loess watershed. J. Hydrol. 2019, 568, 803–815. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, Y.; Qiu, L.; Sivakumar, B.; Zhang, F.; Sun, Y.; Sun, L.; Li, Q.; Voinov, A. Spatiotemporal features of the hydro-biogeochemical cycles in a typical loess gully watershed. Ecol. Indic. 2018, 91, 542–554. [Google Scholar] [CrossRef]
- Harpham, Q.; Hughes, A.; Moore, R. Introductory overview: The OpenMI 2.0 standard for integrating numerical models. Environ. Model. Softw. 2019, 122, 104549. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Abraha, M.; Del Grosso, S.; Robertson, G.; Chen, J. Enhancing the soil and water assessment tool model for simulating N2O emissions of three agricultural systems. Ecosyst. Health Sustain. 2017, 3, e01259. [Google Scholar] [CrossRef] [Green Version]
- Parton, W.; Holland, E.; Del Grosso, S.; Hartman, M.; Martin, R.; Mosier, A.; Ojima, D.; Schimel, D. Generalized model for NOx and N2O emissions from soils. J. Geophys. Res.: Atmos. 2001, 106, 17403–17419. [Google Scholar] [CrossRef]
- Del Grosso, S.; Parton, W.; Mosier, A.; Ojima, D.; Kulmala, A.; Phongpan, S. General model for N2O and N2 gas emissions from soils due to dentrification. Glob. Biogeochem. Cycles 2000, 14, 1045–1060. [Google Scholar] [CrossRef]
- Easton, Z.M.; Fuka, D.R.; Walter, M.T.; Cowan, D.M.; Schneiderman, E.M.; Steenhuis, T.S. Re-conceptualizing the soil and water assessment tool (SWAT) model to predict runoff from variable source areas. J. Hydrol. 2008, 348, 279–291. [Google Scholar] [CrossRef]
- Parton, W.; Mosier, A.; Ojima, D.; Valentine, D.; Schimel, D.; Weier, K.; Kulmala, A.E. Generalized model for N2 and N2O production from nitrification and denitrification. Glob. Biogeochem. Cycles 1996, 10, 401–412. [Google Scholar] [CrossRef]
- Mosier, A.; Doran, J.; Freney, J. Managing soil denitrification. J. Soil Water Conserv. 2002, 57, 505–512. [Google Scholar]
- Wagena, M.B.; Collick, A.S.; Ross, A.C.; Najjar, R.G.; Rau, B.; Sommerlot, A.R.; Fuka, D.R.; Kleinman, P.J.; Easton, Z.M. Impact of climate change and climate anomalies on hydrologic and biogeochemical processes in an agricultural catchment of the Chesapeake Bay watershed, USA. Sci. Total Environ. 2018, 637, 1443–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.; Lee, X.; Griffis, T.J.; Baker, J.M.; Turner, P.A. A modeling study of direct and indirect N2O emissions from a representative catchment in the US Corn Belt. Water Resour. Res. 2018, 54, 3632–3653. [Google Scholar] [CrossRef]
- Parton, W.J.; Ojima, D.S.; Cole, C.V.; Schimel, D.S. A general model for soil organic matter dynamics: Sensitivity to litter chemistry, texture and management. Quant. Modeling Soil Form. Process. 1994, 39, 147–167. [Google Scholar]
- Gao, X.; Ouyang, W.; Hao, Z.; Xie, X.; Lian, Z.; Hao, X.; Wang, X. SWAT-N2O coupler: An integration tool for soil N2O emission modeling. Environ. Model. Softw. 2019, 115, 86–97. [Google Scholar] [CrossRef]
- Xie, X.; Cui, Y. Development and test of SWAT for modeling hydrological processes in irrigation districts with paddy rice. J. Hydrol. 2011, 396, 61–71. [Google Scholar] [CrossRef]
- Bhanja, S.N.; Wang, J.; Shrestha, N.K.; Zhang, X. Microbial kinetics and thermodynamic (MKT) processes for soil organic matter decomposition and dynamic oxidation-reduction potential: Model descriptions and applications to soil N2O emissions. Environ. Pollut. 2019, 247, 812–823. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B: Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, J.; Wang, L.; Yang, L. Advance in a terrestrial biogeochemical model—DNDC model. Acta Ecol. Sin. 2011, 31, 91–96. [Google Scholar] [CrossRef]
- Pohlert, T.; Huisman, J.; Breuer, L.; Frede, H.-G. Integration of a detailed biogeochemical model into SWAT for improved nitrogen predictions—Model development, sensitivity, and GLUE analysis. Ecol. Model. 2007, 203, 215–228. [Google Scholar] [CrossRef]
- Marzadri, A.; Dee, M.M.; Tonina, D.; Bellin, A.; Tank, J.L. Role of surface and subsurface processes in scaling N2O emissions along riverine networks. Proc. Natl. Acad. Sci. USA 2017, 114, 4330–4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, U.; Shrestha, N.K.; Biswas, A.; Wagner-Riddle, C.; Yang, W.; Prasher, S.; Rudra, R.; Daggupati, P. A Review of Ongoing Advancements in Soil and Water Assessment Tool (SWAT) for Nitrous Oxide (N2o) Modeling. Atmosphere 2020, 11, 450. https://doi.org/10.3390/atmos11050450
Ghimire U, Shrestha NK, Biswas A, Wagner-Riddle C, Yang W, Prasher S, Rudra R, Daggupati P. A Review of Ongoing Advancements in Soil and Water Assessment Tool (SWAT) for Nitrous Oxide (N2o) Modeling. Atmosphere. 2020; 11(5):450. https://doi.org/10.3390/atmos11050450
Chicago/Turabian StyleGhimire, Uttam, Narayan Kumar Shrestha, Asim Biswas, Claudia Wagner-Riddle, Wanhong Yang, Shiv Prasher, Ramesh Rudra, and Prasad Daggupati. 2020. "A Review of Ongoing Advancements in Soil and Water Assessment Tool (SWAT) for Nitrous Oxide (N2o) Modeling" Atmosphere 11, no. 5: 450. https://doi.org/10.3390/atmos11050450
APA StyleGhimire, U., Shrestha, N. K., Biswas, A., Wagner-Riddle, C., Yang, W., Prasher, S., Rudra, R., & Daggupati, P. (2020). A Review of Ongoing Advancements in Soil and Water Assessment Tool (SWAT) for Nitrous Oxide (N2o) Modeling. Atmosphere, 11(5), 450. https://doi.org/10.3390/atmos11050450