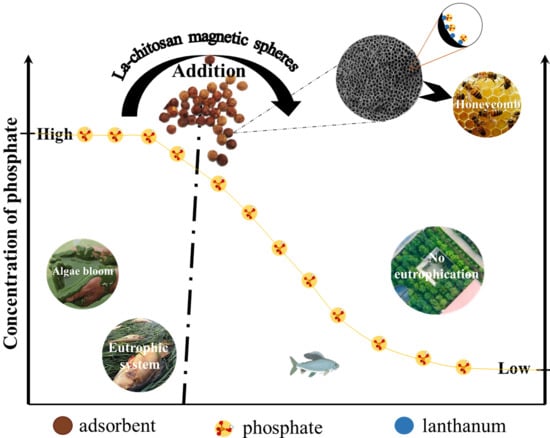
Enhanced Phosphate Removal from Water by Honeycomb-Like Microporous Lanthanum-Chitosan Magnetic Spheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Lanthanum-Chitosan Magnetic Spheres
2.3. Characterization of La-Chitosan Magnetic Spheres
2.4. Phosphate Adsorption Experiments
2.4.1. Adsorption Kinetics
2.4.2. Adsorption Isotherm
2.4.3. Thermodynamic Analysis
2.4.4. Conditional Factor Experiments
3. Results and Discussion
3.1. Characterization
3.1.1. SEM Analysis
3.1.2. VSM Analysis
3.1.3. X-ray Diffraction Analysis
3.1.4. FTIR Analysis
3.2. Adsorption Experiments
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherm
3.2.3. Thermodynamic Analysis
3.2.4. Effect of La/CS Ratios
3.2.5. The Effect of Dosage
3.2.6. The Effect of pH
3.2.7. The Effect of Coexisting Anions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahdavi, S.; Akhzari, D. The removal of phosphate from aqueous solutions using two nano-structures: Copper oxide and carbon tubes. Clean Technol. Environ. Policy 2016, 18, 817–827. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Tu, Y.; You, C. Phosphorus adsorption onto green synthesized nano-bimetal ferrites: Equilibrium, kinetic and thermodynamic investigation. Chem. Eng. J. 2014, 251, 285–292. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Q.; Peng, L.; Lei, M.; Song, H.; Tie, B.; Gu, J. La-EDTA coated Fe3O4 nanomaterial: Preparation and application in removal of phosphate from water. J. Environ. Sci. 2013, 25, 413–418. [Google Scholar] [CrossRef]
- Lürling, M.; Van, O.F. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res. 2013, 47, 6527–6537. [Google Scholar] [CrossRef] [PubMed]
- Jabari, P.; Munz, G.; Yuan, Q.; Oleszkiewicz, J.A. Free nitrous acid inhibition of biological phosphorus removal in integrated fixed-film activated sludge (IFAS) system. Chem. Eng. J. 2016, 287, 38–46. [Google Scholar] [CrossRef]
- Zheng, X.; Pan, J.; Zhang, F.; Liu, E.; Shi, W.; Yan, Y. Fabrication of free-standing bio-template mesoporous hybrid film for high and selective phosphate removal. Chem. Eng. J. 2016, 284, 879–887. [Google Scholar] [CrossRef]
- Dithmer, L.; Nielsen, U.G.; Lundberg, D.; Reitzel, K. Influence of dissolved organic carbon on the efficiency of P sequestration by a lanthanum modified clay. Water Res. 2016, 97, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, F.; He, J.; Xu, H.; Cui, F. Robust phosphate capture over inorganic adsorbents derived from lanthanum metal organic frameworks. Chem. Eng. J. 2017, 326, 1086–1094. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, X.; Pan, B.; Zhang, W.; Hua, M.; Lv, L.; Zhang, W. Preferable removal of phosphate from water using hydrous zirconium oxide-based nanocomposite of high stability. J. Hazard. Mater. 2015, 284, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, Y.; Zhou, Q.; Kan, J.; Wang, Y. High-Performance Removal of Phosphate from Water by Graphene Nanosheets Supported Lanthanum Hydroxide Nanoparticles. Water Air Soil Pollut. 2014, 225. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, R.K. Sequencing batch reactor technology for biological wastewater treatment: A review. Asia-Pac. J. Chem. Eng. 2011, 6, 3–13. [Google Scholar] [CrossRef]
- Lai, L.; Xie, Q.; Chi, L.; Gu, W.; Wu, D. Adsorption of phosphate from water by easily separable Fe3O4@SiO2 core/shell magnetic nanoparticles functionalized with hydrous lanthanum oxide. J. Colloid Interface Sci. 2016, 465, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res. 2013, 47, 5018–5026. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Pan, G.; Chen, H.; Tian, B. Phosphorus fixation in lake sediments using LaCl3-modified clays. Ecol. Eng. 2009, 35, 1599–1602. [Google Scholar] [CrossRef]
- Tu, C.; Wang, S.; Qiu, W.; Xie, R.; Hu, B. Phosphorus Removal from Aqueous Solution by Adsorption onto La-modified Clinoptilolite. MATEC Web Conf. 2016, 67, 07013. [Google Scholar] [CrossRef]
- Zong, E.; Liu, X.; Wang, J.; Yang, S.; Jiang, J.; Fu, S. Facile preparation and characterization of lanthanum-loaded carboxylated multi-walled carbon nanotubes and their application for the adsorption of phosphate ions. J. Mater. Sci. 2017, 52, 7294–7310. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen (adsorption in solution). Z. Phys. Chem. 1906, 57, 384–470. [Google Scholar]
- Butt, H.J.; Graf, K.; Kappl, M. Physical and Chemistry of Interfaces; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Ma, H.; Pu, S.; Ma, J.; Yan, C.; Zinchenko, A.; Pei, X.; Chu, W. Formation of multi-layered chitosan honeycomb spheres via breath-figure-like approach in combination with co-precipitation processing. Mater. Lett. 2018, 211, 91–95. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, S.; Yu, J.; Shu, Z. Novel hollow microspheres of hierarchical zinc-aluminum layered double hydroxides and their enhanced adsorption capacity for phosphate in water. J. Hazard. Mater. 2011, 192, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xu, Y.; Yu, H.; Xin, X.; Wei, Q.; Du, B. Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron–aluminum pillared bentonites. J. Hazard. Mater. 2010, 179, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Haghseresht, F.; Wang, S.; Do, D.D. A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl. Clay Sci. 2009, 46, 369–375. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Liu, J.; Zhang, L. Phosphorus removal from wastewater using nano-particulates of hydrated ferric oxide doped activated carbon fiber prepared by Sol–Gel method. Chem. Eng. J. 2012, 200–202, 619–626. [Google Scholar] [CrossRef]
- Huang, W.; Li, D.; Liu, Z.; Tao, Q.; Zhu, Y.; Yang, J.; Zhang, Y. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La (OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents. Chem. Eng. J. 2014, 236, 191–201. [Google Scholar] [CrossRef]
- Kuroki, V.; Bosco, G.E.; Fadini, P.S.; Mozeto, A.A.; Cestari, A.R. Use of a La (III)-modified bentonite for effective phosphate removal from aqueous media. J. Hazard. Mater. 2014, 274, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Genz, A.; Kornmüller, A.; Jekel, M. Advanced phosphorus removal from membrane filtrates by adsorption on activated aluminium oxide and granulated ferric hydroxide. Water Res. 2004, 38, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Zamparas, M.; Gianni, A.; Stathi, P.; Deligiannakis, Y.; Zacharias, I. Removal of phosphate from natural waters using innovative modified bentonites. Appl. Clay Sci. 2012, 62–63, 101–106. [Google Scholar] [CrossRef]
- Huang, W.; Li, D.; Yang, J.; Liu, Z.; Zhu, Y.; Tao, Q.; Xu, K.; Li, J.; Zhang, Y. One-pot synthesis of Fe (III)-coordinated diamino-functionalized mesoporous silica: Effect of functionalization degrees on structures and phosphate adsorption. Microporous Mesoporous Mater. 2013, 170, 200–210. [Google Scholar] [CrossRef]











(mg·L−1) | (mg g−1) | Pseudo-First-Order | Pseudo-Second-Order | Intra-Particle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
(mg g−1) | (min−1) | R2 | (mg g−1) | (g mg−1 min−1) | R2 | (g mg−1 min−1) | (g mg−1 min−1) | R2 | ||
| 20 | 54.08 | 41.69 | 0.01 | 0.97 | 59.52 | 0.02 | 0.99 | 2.72 | 0.56 | 0.97 |
| Adsorbents | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|
| qmax (mg P·g−1) | KL (L·mg−1) | RL | R2 | KF | 1/n | R2 | |
| La-chitosan magnetic spheres | 27.78 | 1.27 | 0.0379 | 0.99 | 15.16 | 0.54 | 0.98 |
| Temperature (°C) | b (L/mol) | |||
|---|---|---|---|---|
| 15 | 646,695 | −32.04 | −0.1615 | −77.73 |
| 25 | 90,932 | −8.29 | ||
| 35 | 55,181 | −27.96 | ||
| 45 | 26,061 | −26.88 |
| Type of Adsorbents | qm (mg P·g−1) | Isotherm Model | Reference |
|---|---|---|---|
| La-chitosan magnetic spheres | 27.78 | Langmuir | This work |
| Fe3O4@SiO2 with La2O3 | 27.8 | Langmuir | [14] |
| Phoslock® (lanthanum-modified bentonite) | 9.5–10.5 | Langmuir | [25] |
| NT-25La | 14.0 | Langmuir | [28] |
| Activated aluminium oxide | 13.8 | Langmuir | [29] |
| Fe (III)-modified bentonite | 11.2 | Langmuir | [30] |
| Al (III)-modified bentonite | 12.7 | Langmuir | [31] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, R.; Shen, L.-J.; Zhang, Y.-Y.; Dai, D.-Y.; Zheng, X.; Liao, L.-W.; Wang, L.; Shi, L. Enhanced Phosphate Removal from Water by Honeycomb-Like Microporous Lanthanum-Chitosan Magnetic Spheres. Water 2018, 10, 1659. https://doi.org/10.3390/w10111659
Cheng R, Shen L-J, Zhang Y-Y, Dai D-Y, Zheng X, Liao L-W, Wang L, Shi L. Enhanced Phosphate Removal from Water by Honeycomb-Like Microporous Lanthanum-Chitosan Magnetic Spheres. Water. 2018; 10(11):1659. https://doi.org/10.3390/w10111659
Chicago/Turabian StyleCheng, Rong, Liang-Jie Shen, Ying-Ying Zhang, Dan-Yang Dai, Xiang Zheng, Long-Wen Liao, Lei Wang, and Lei Shi. 2018. "Enhanced Phosphate Removal from Water by Honeycomb-Like Microporous Lanthanum-Chitosan Magnetic Spheres" Water 10, no. 11: 1659. https://doi.org/10.3390/w10111659
APA StyleCheng, R., Shen, L. -J., Zhang, Y. -Y., Dai, D. -Y., Zheng, X., Liao, L. -W., Wang, L., & Shi, L. (2018). Enhanced Phosphate Removal from Water by Honeycomb-Like Microporous Lanthanum-Chitosan Magnetic Spheres. Water, 10(11), 1659. https://doi.org/10.3390/w10111659