Diel Variability of pCO2 and CO2 Outgassing from the Lower Mississippi River: Implications for Riverine CO2 Outgassing Estimation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Hourly River Discharge, Water Conditions, and Atmospheric Measurements
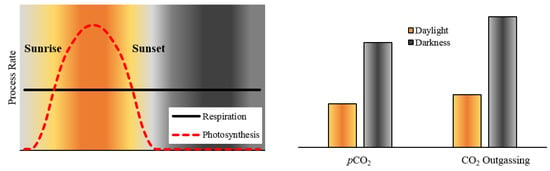
3.2. Diel pCO2 Measurements
3.3. CO2 Outgassing Estimates
4. Discussion
4.1. Seasonal pCO2 Differences
4.2. Biological Processes Influencing Diel pCO2 Variation
4.3. Implications for Carbon Outgassing Estimates and Future Research Needs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butman, D.; Raymond, P.A. Significant efflux of carbon dioxide from streams and rivers in the United States. Nat. Geosci. 2011, 4, 839–842. [Google Scholar] [CrossRef]
- Cole, J.J.; Caraco, N.F. Carbon in catchments: Connecting terrestrial carbon losses with aquatic metabolism. Mar. Freshw. Res. 2001, 52, 101–110. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richey, J.E. Pathways of atmospheric CO2 through fluvial systems. In Scientific Committee on Problems of the Environment (SCOPE)/United Nations Environment Programme (UNEP)—The Global Carbon Cycle: Integrating Humans, Climate, and the Natural World; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Richey, J.E.; Melack, J.M.; Aufdenkampe, A.K.; Ballester, V.M.; Hess, L.L. Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2. Nature 2002, 416, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Lu, X.X.; Richey, J.E.; Sun, H.; Han, J.; Yu, R.; Liao, S.; Yi, Q. Long-term spatial and temporal variation of CO2 partial pressure in the Yellow River, China. Biogeosciences 2015, 12, 921–932. [Google Scholar] [CrossRef]
- Lauerwald, R.; Laruelle, G.G.; Hartmann, J.; Ciais, P.; Regnier, P.A.G. Spatial patterns in CO2 evasion from the global river network. Glob. Biogeochem. Cycles 2015, 29, 534–554. [Google Scholar] [CrossRef]
- Abril, G.; Bouillon, S.; Darchambeau, F.; Teodoru, C.R.; Marwick, T.R.; Tamooh, F.; Ochieng Omengo, F.; Geeraert, N.; Deirmendjian, L.; Polsenaere, P.; et al. Technical Note: Large overestimation of pCO2 calculated from pH and alkalinity in acidic, organic-rich freshwaters. Biogeosciences 2015, 12, 67–78. [Google Scholar] [CrossRef]
- Meybeck, M. Carbon, nitrogen, and phosphorus transport by world rivers. Am. J. Sci. 1982, 282, 401–450. [Google Scholar] [CrossRef]
- Meybeck, M. Riverine transport of atmospheric carbon–sources, global typology and budget. Water Air Soil Pollut. 1993, 70, 443–463. [Google Scholar] [CrossRef]
- Aitkenhead, J.A.; McDowell, W.H. Soil C:N ratio as a predictor of annual riverine DOC flux at local and global scales. Glob. Biogeochem. Cycles 2000, 14, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Peng, C.; Wang, M.; Xue, W.; Zhang, K.; Wang, K.; Shi, G.; Zhu, Q. The carbon flux of global rivers: A re-evaluation of amount and spatial patterns. Ecol. Indic. 2017, 80, 40–51. [Google Scholar] [CrossRef]
- Hélie, J.-F.; Hillaire-Marcel, C.; Rondeau, B. Seasonal changes in sources and fluxes of dissolved inorganic carbon through the St. Lawrence River—Isotopic and chemical constraint. Chem. Geol. 2002, 186, 117–138. [Google Scholar] [CrossRef]
- Ran, L.; Lu, X.X.; Liu, S. Dynamics of riverine CO2 in the Yangtze River fluvial network and their implications for carbon evasion. Biogeosciences 2017, 14, 2183–2198. [Google Scholar] [CrossRef]
- Raymond, P.A.; Caraco, N.F.; Cole, J.J. Carbon dioxide concentration and atmospheric flux in the Hudson River. Estuaries 1997, 20, 381–390. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, M.; Wang, M.; Cai, W.-J.; Wang, L.; Yu, Z. The spatiotemporal distribution of dissolved inorganic and organic carbon in the main stem of the Changjiang (Yangtze) River and the effect of the Three Gorges Reservoir. J. Geophys. Res. Biogeosci. 2014, 119, 741–757. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.V.; Darchambeau, F.; Lambert, T.; Bouillon, S.; Morana, C.; Brouyère, S.; Hakoun, V.; Jurado, A.; Tseng, H.C.; Descy, J.P.; et al. Effects of agricultural land use on fluvial carbon dioxide, methane and nitrous oxide concentrations in a large European river, the Meuse (Belgium). Sci. Total Environ. 2018, 610–611, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Lynch, J.K.; Beatty, C.M.; Seidel, M.P.; Jungst, L.J.; DeGrandpre, M.D. Controls of riverine CO2 over an annual cycle determined using direct, high temporal resolution pCO2 measurements. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Yang, R.; Chen, B.; Liu, H.; Liu, Z.; Yan, H. Carbon sequestration and decreased CO2 emission caused by terrestrial aquatic photosynthesis: Insights from diel hydrochemical variations in an epikarst spring and two spring-fed ponds in different seasons. Appl. Geochem. 2015, 63, 248–260. [Google Scholar] [CrossRef]
- Yates, K.K.; Dufore, C.; Smiley, N.; Jackson, C.; Halley, R.B. Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay. Mar. Chem. 2007, 104, 110–124. [Google Scholar] [CrossRef]
- Spafford, L.; Risk, D. Spatiotemporal Variability in Lake-Atmosphere Net CO2 Exchange in the Littoral Zone of an Oligotrophic Lake. J. Geophys. Res. Biogeosci. 2018, 123, 1260–1276. [Google Scholar] [CrossRef]
- Liu, H.P.; Zhang, Q.Y.; Katul, G.G.; Cole, J.J.; Chapin, F.S.; MacIntyre, S. Large CO2 effluxes at night and during synoptic weather events significantly contribute to CO2 emissions from a reservoir. Environ. Res. Lett. 2016, 11, 8. [Google Scholar] [CrossRef]
- Xu, Y.J.; DelDuco, E.M. Unravelling the relative contribution of dissolved carbon by the Red River to the Atchafalaya River. Water 2017, 9, 871. [Google Scholar] [CrossRef]
- Reiman, J.R.; Xu, Y.J. A recent update in carbon transport: The Mississippi-Atchafalaya River System. In Proceedings of the 2018 State of the Coast Conference, New Orleans, LA, USA, 30 May–1 June 2018. [Google Scholar] [CrossRef]
- Cai, Y.H.; Guo, L.D.; Wang, X.R.; Aiken, G. Abundance, stable isotopic composition, and export fluxes of DOC, POC, and DIC from the Lower Mississippi River during 2006–2008. J. Geophys. Res. Biogeosci. 2015, 120, 2273–2288. [Google Scholar] [CrossRef]
- Ren, W.; Tian, H.; Cai, W.-J.; Lohrenz, S.E.; Hopkinson, C.S.; Huang, W.-J.; Yang, J.; Tao, B.; Pan, S.; He, R. Century-long increasing trend and variability of dissolved organic carbon export from the Mississippi River basin driven by natural and anthropogenic forcing. Glob. Biogeochem. Cycles 2016, 30, 1288–1299. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Wysocki, L.A.; Stewart, M.; Filley, T.R.; McKee, B.A. Temporal variability in terrestrially-derived sources of particulate organic carbon in the lower Mississippi River and its upper tributaries. Geochim. Cosmochim. Acta 2007, 71, 4425–4437. [Google Scholar] [CrossRef]
- Dubois, K.D.; Lee, D.; Veizer, J. Isotopic constraints on alkalinity, dissolved organic carbon, and atmospheric carbon dioxide fluxes in the Mississippi River. J. Geophys. Res. Biogeosci. 2010, 115, 2156–2202. [Google Scholar] [CrossRef]
- Shen, Y.; Fichot, C.G.; Benner, R. Floodplain influence on dissolved organic matter composition and export from the Mississippi-Atchafalaya River system to the Gulf of Mexico. Limnol. Oceanogr. 2012, 57, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- BryantMason, A.; Xu, Y.J.; Altabet, M. Isotopic signature of nitrate in river waters of the lower Mississippi and its distributary, the Atchafalaya. Hydrol. Process. 2013, 27, 2840–2850. [Google Scholar] [CrossRef]
- USACE. The 2013 Mississippi River Hydrographic Survey Book; U.S. Army Corps of Engineers District: New Orleans, LA, USA, 2013; p. 91. Available online: http://www.mvn.usace.army.mil/Portals/56/docs/engineering/Geospatial/MRHB_2013/PDF/MRHB_2013.pdf Survey charts (accessed on 5 November 2018).
- Dodds, W.K.; Veach, A.M.; Ruffing, C.M.; Larson, D.M.; Fischer, J.L.; Costigan, K.H. Abiotic controls and temporal variability of river metabolism: Multiyear analyses of Mississippi and Chattahoochee River data. Freshw. Sci. 2013, 32, 1073–1087. [Google Scholar] [CrossRef]
- Green, R.F.; Bianchi, T.S.; Dagg, M.J.; Walker, N.D.; Breed, G.A. An organic carbon budget for the Mississippi River turbidity plume and plume contributions to air-sea CO2 fluxes and bottom water hypoxia. Estuaries Coasts 2006, 29, 579–597. [Google Scholar] [CrossRef]
- Guo, X.; Cai, W.-J.; Huang, W.-J.; Wang, Y.; Chen, F.; Murrell, M.C.; Lohrenz, S.E.; Jiang, L.-Q.; Dai, M.; Hartmann, J.; et al. Carbon dynamics and community production in the Mississippi River plume. Limnol. Oceanogr. 2012, 57, 1–17. [Google Scholar] [CrossRef]
- Cai, W.-J.; Wang, Y. The chemistry, fluxes, and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha Rivers, Georgia. Limnol. Oceanogr. 1998, 43, 657–668. [Google Scholar] [CrossRef] [Green Version]
- National Ocean & Atmospheric Administration Earth System Research Laboratory Global Monitoring Division Website. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/data.html (accessed on 12 June 2018).
- Ward, H.C.; Kotthaus, S.; Grimmond, C.S.; Bjorkegren, A.; Wilkinson, M.; Morrison, W.T.; Evans, J.G.; Morison, J.I.; Iamarino, M. Effects of urban density on carbon dioxide exchanges: Observations of dense urban, suburban and woodland areas of southern England. Environ. Pollut. 2015, 198, 186–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imasu, R.; Tanabe, Y. Diurnal and seasonal variations of carbon dioxide (CO2) concentrations in urban, suburban, and rural areas around Tokyo. Atmosphere 2018, 9, 367. [Google Scholar] [CrossRef]
- Alin, S.R.; de Fátima, F.L.; Rasera, M.; Salimon, C.I.; Richey, J.E.; Holtgrieve, G.W.; Krusche, A.V.; Snidvongs, A. Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. J. Geophys. Res. Biogeosci. 2011, 116. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.F. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Hope, D.; Palmer, S.M.; Billett, M.F.; Dawson, J.J.C. Variations in dissolved CO2 and CH4 in a first-order stream and catchment: An investigation of soil-stream linkages. Hydrol. Process. 2004, 18, 3255–3275. [Google Scholar] [CrossRef]
- Finlay, J.C. Controls of streamwater dissolved inorganic carbon dynamics in a forested watershed. Biogeochemistry 2003, 62, 231–252. [Google Scholar] [CrossRef]
- Barth, J.A.; Veizer, J. Carbon cycle in St. Lawrence aquatic ecosystems at Cornwall (Ontario), Canada: Seasonal and spatial variations. Chem. Geol. 1999, 159, 107–128. [Google Scholar] [CrossRef]
- Vesala, T.; Huotari, J.; Rannik, Ü.; Suni, T.; Smolander, S.; Sogachev, A.; Launiainen, S.; Ojala, A. Eddy covariance measurements of carbon exchange and latent and sensible heat fluxes over a boreal lake for a full open-water period. J. Geophys. Res. Atmos. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Vale, R.; Santana, R.; Gomes, A.C.; Tóta, J. Increased nocturnal CO2 concentration during breeze circulation events in a tropical reservoir. Ambient. Água Interdisc. J. Appl. Sci. 2018. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Fellows, C.S.; Tank, J.L.; Grimm, N.B.; Webster, J.R.; Hamilton, S.K.; Martí, E.; Ashkenas, L.; Bowden, W.B.; Dodds, W.K.; et al. Inter-biome comparison of factors controlling stream metabolism. Freshw. Biol. 2001, 46, 1503–1517. [Google Scholar] [CrossRef] [Green Version]
- Bott, T.L.; Montgomery, D.S.; Newbold, J.D.; Arscott, D.B.; Dow, C.L.; Aufdenkampe, A.K.; Jackson, J.K.; Kaplan, L.A. Ecosystem metabolism in streams of the Catskill Mountains (Delaware and Hudson River watersheds) and Lower Hudson Valley. J. N. Am. Benthol. Soc. 2006, 25, 1018–1044. [Google Scholar] [CrossRef]
- Naiman, R.J. The annual pattern and spatial distribution of aquatic oxygen metabolism in boreal forest watersheds. Ecol. Monogr. 1983, 53. [Google Scholar] [CrossRef]
- Dawson, J.J.C.; Billett, M.F.; Hope, D. Diurnal variations in the carbon chemistry of two acidic peatland streams in north-east Scotland. Freshw. Biol. 2001, 46, 1309–1322. [Google Scholar] [CrossRef]
- Jähne, B.; Münnich, K.O.; Bösinger, R.; Dutzi, A.; Huber, W.; Libner, P. On the parameters influencing air-water gas exchange. J. Geophys. Res. 1987, 92, 1937–1949. [Google Scholar] [CrossRef]
- Schubert, M.; Forster, R.M. Sources of variability in the factors used for modelling primary productivity in eutrophic waters. Hydrobiologia 1997, 349, 75–85. [Google Scholar] [CrossRef]
- Helbling, E.W.; Banaszak, A.T.; Villafane, V.E. Global change feed-back inhibits cyanobacterial photosynthesis. Sci. Rep. 2015, 5, 14514. [Google Scholar] [CrossRef] [Green Version]
- Sand-Jensen, K.; Pedersen, M.F.; Laurentius, S. Photosynthetic use of inorganic carbon among primary and secondary water plants in streams. Freshw. Biol. 1992, 27, 283–293. [Google Scholar] [CrossRef]
- Rich, S.M.; Pedersen, O.; Ludwig, M.; Colmer, T.D. Shoot atmospheric contact is of little importance to aeration of deeper portions of the wetland plant Meionectes brownii; submerged organs mainly acquire O2 from the water column or produce it endogenously in underwater photosynthesis. Plant Cell Environ. 2013, 36, 213–223. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Sand-Jensen, K. Light attenuation and photosynthesis of aquatic plant communities. Limnol. Oceanogr. 1998, 43, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, O.; Colmer, T.D.; Sand-Jensen, K. Underwater photosynthesis of submerged plants—Recent advances and methods. Front. Plant Sci. 2013, 4, 140. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.E.; Rabalais, N.N.; Alexander, R.B.; McIsaac, G.; Howarth, R.W. Characterization of nutrient, organic carbon, and sediment loads and concentrations from the Mississippi River into the northern Gulf of Mexico. Estuaries Coasts 2007, 30, 773–790. [Google Scholar] [CrossRef]
- Borges, A.V.; Abril, G.; Darchambeau, F.; Teodoru, C.R.; Deborde, J.; Vidal, L.O.; Lambert, T.; Bouillon, S. Divergent biophysical controls of aquatic CO2 and CH4 in the World’s two largest rivers. Sci. Rep. 2015, 5, 15614. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.V.; Darchambeau, F.; Teodoru, C.R.; Marwick, T.R.; Tamooh, F.; Geeraert, N.; Omengo, F.O.; Guérin, F.; Lambert, T.; Morana, C.; et al. Globally significant greenhouse gas emissions from African inland waters. Nat. Geosci. 2015, 8, 637–642. [Google Scholar] [CrossRef]
- Descy, J.P.; Darchambeau, F.; Lambert, T.; Stoyneva, M.P.; Bouillon, S.; Borges, A.V. Phytoplankton dynamics in the Congo River. Freshw. Biol. 2017, 62, 87–101. [Google Scholar] [CrossRef]
- Townsend, S.A.; Webster, I.T.; Schult, J.H. Metabolism in a groundwater-fed river system in the Australian wet/dry tropics: Tight coupling of photosynthesis and respiration. J. N. Am. Benthol. Soc. 2011, 30, 603–620. [Google Scholar] [CrossRef]





| Date | Morning | R2 (p-Value) | Evening | R2 (p-Value) |
|---|---|---|---|---|
| 11 May | pCO2 = −42.14H + 2421 | 0.95 (0.0009) | pCO2 = 39.67H + 1130 | 0.90 (0.0036) |
| 17 May | pCO2 = −21.99H + 2479 | 0.98 (0.0081) | pCO2 = 9.016H + 2025 | 0.40 (0.1782) |
| 13 Aug | pCO2 = −38.54H + 1254 | 0.86 (0.0709) | pCO2 = 16.93H + 467.0 | 0.86 (0.0081) |
| 15 Aug | pCO2 = −37.78H + 1250 | 0.99 (0.0033) | pCO2 = 24.96H + 357.1 | 0.93 (0.0018) |
| 11 May | FCO2 = −0.3285H + 14.58 | 0.97 (0.0010) | FCO2 = 0.2859H + 4.923 | 0.91 (0.0028) |
| 17 May | FCO2 = −0.1767H + 13.78 | 0.99 (0.0016) | FCO2 = 0.06883H + 10.22 | 0.45 (0.1434) |
| 13 Aug | FCO2 = −0.2178H + 4.743 | 0.86 (0.0700) | FCO2 = 0.09467H + 0.3138 | 0.86 (0.0077) |
| 15 Aug | FCO2 = −0.2113H + 4.668 | 0.99 (0.0037) | FCO2 = 0.1379H − 0.2888 | 0.93 (0.0018) |
| pCO2 | Tw | DO | pH | N | Ta | u | SR | Q | |
|---|---|---|---|---|---|---|---|---|---|
| pCO2 | 1.00 | −0.92 | - | −0.98 | 0.46 | - | - | - | 0.93 |
| Tw | 1.00 | - | 0.96 | −0.46 | - | - | - | −0.99 | |
| DO | 1.00 | - | - | - | - | - | - | ||
| pH | 1.00 | −0.47 | - | - | 0.76 | −0.97 | |||
| N | 1.00 | - | 0.48 | - | 0.49 | ||||
| Ta | 1.00 | 0.63 | 0.86 | - | |||||
| u | 1.00 | 0.60 | 0.40 | ||||||
| SR | 1.00 | - | |||||||
| Q | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiman, J.H.; Xu, Y.J. Diel Variability of pCO2 and CO2 Outgassing from the Lower Mississippi River: Implications for Riverine CO2 Outgassing Estimation. Water 2019, 11, 43. https://doi.org/10.3390/w11010043
Reiman JH, Xu YJ. Diel Variability of pCO2 and CO2 Outgassing from the Lower Mississippi River: Implications for Riverine CO2 Outgassing Estimation. Water. 2019; 11(1):43. https://doi.org/10.3390/w11010043
Chicago/Turabian StyleReiman, Jeremy H., and Y. Jun Xu. 2019. "Diel Variability of pCO2 and CO2 Outgassing from the Lower Mississippi River: Implications for Riverine CO2 Outgassing Estimation" Water 11, no. 1: 43. https://doi.org/10.3390/w11010043
APA StyleReiman, J. H., & Xu, Y. J. (2019). Diel Variability of pCO2 and CO2 Outgassing from the Lower Mississippi River: Implications for Riverine CO2 Outgassing Estimation. Water, 11(1), 43. https://doi.org/10.3390/w11010043