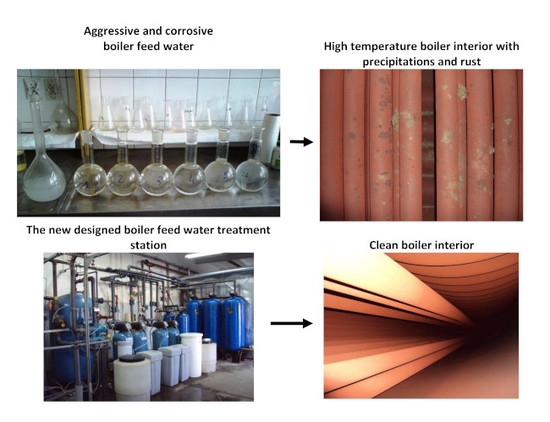
Treatment Method Assessment of the Impact on the Corrosivity and Aggressiveness for the Boiler Feed Water †
Abstract
:1. Introduction
2. Material and Methods
2.1. Analytical Methods
- —sulphates concentration (VI) [mg/dm3],
- [Cl−]—chlorides concentration [mg/dm3],
- —bicarbonates concentration [mg/dm3],
- —carbonates concentration [mg/dm3].
- —sulphates concentration (VI) [mg/dm3],
- [Cl−]—chlorides concentration [mg/dm3],
- —sodium concentration [mg/dm3],
- —initial dissolved oxygen content [mg/dm3],
- T—temperature [0C],
- alk—alkalinity [mg CaCO3/ dm3],
- HRT—hydraulic retention time [days].
- pH—real reaction of water,
- pHs—pH value of the water in the saturated state.
- L—the solubility product of calcium carbonate CaCO3,
- K2—the second carbonic acid dissociation constant.
2.2. Technological Methods
- Water flow chart—20 m3/h,
- Water flow rate—1–1.5 m/s,
- Primary aeration: open tank volume—3 m3, retention time—10 min, non-pressure aerators with air flow 0.5–1 Nm2/h,
- Quartz sand filtration: two filters DN800, H = 1.8 m, V load = 17 m3/m2 h,
- Cation exchange: five columns with weak cation exchange resign, DN300, H = 1.5 m, Q = 4 m3/h.
- -
- Pre-aeration of raw water taken from the well. For experiments, a new aeration system was designed, including an in-line mixer combined with compressor with air flow of about 2–3 Nm2/h. Samples of aerated water were taken after flow compensation and the escape of air bubbles from the analysed water after venting valves.
- -
- Use of weak base anion exchange resin, which eliminates from the water disturbing ions of strong acids: SO42− and Cl−. Such mass additionally increases the water pH and influences the aggressiveness indexes.
- -
- Additional (extra) aeration of treated water. The process was planned after water deionization. In-line aerators combined with a compressor with air flow of 4–5 Nm2/h were used.
- -
- Extra deaerator (degassing system) as the last process of water purification. For experiments, a thermal pressure deaerator was used. Degassing occurred at temperatures above 150 °C and operating pressures around 6 to 8 bar.
- Water flow chart—20 m3/h,
- Water flow rate—1–1.5 m/s,
- Pre-aeration: in-line static mixer DN80 combined with compressor with air flow of about 2–3 Nm2/h,
- Quartz sand filtration: two filters DN800, H = 1.8 m, V load = 17 m3/m2 h,
- Cation exchange: five columns with new weak cation exchange resign, DN300, H = 1.5 m, Q = 4 m3/h,
- Anion exchange: five columns with weak base anion exchange resin, DN300, H = 1.8 m, Q = 4 m3/h,
- Extra aeration: two in-line static mixers DN80 combined with compressor with air flow of about 5–6 Nm2/h,
- Thermal pressure deaerator: DN1000, H = 2 m, V = 2 m3.
3. Results and Discussion
4. Summary and Conclusions
- Studied boiler feed water has special requirements and must be free of most impurities, corrosivity and aggressiveness. Such a purification level required highly-efficient treatment, such as pre-aeration, filtration, cation exchange, anion exchange, extra aeration, and extra degassing.
- The estimation of O2 and CO2 concentration in water was important in the conducted research. After modernization, the oxygen concentration changed from 0.5 mg/dm3 in raw water to the maximal level of 3.5 mg/dm3 after aeration and 2.6 mg/dm3 after filtration. CO2 changed gradually from over 90 mg/dm3 to 6 mg/dm3 in deionized water. Oxygen and free carbon dioxide concentrations dropped to 0.0 mg/dm3 after extra aeration and extra degassing.
- In the conducted studies, the indirect method of water aggressiveness and corrosivity assessment was applied using mathematical calculation of the Langelier Saturation Index (LSI), the Ryznar Stability Index (RI), the Larson–Skold Index (LI), and the Singley Index (SI).
- In this study, the Larson–Skold Index calculations proved that water treatment processes after modernization did not have any influence on water corrosion. Purified water in every individual purification process was neither corrosive nor aggressive.
- The Singley Index also describes water corrosivity, but its formula contains more variables than LI. The results showed that water was corrosive from the first treatment up to ion exchange. Extra aeration reduced water corrosion to SI = 0.75 and degassing improved the effect. It made water neither corrosive nor aggressive by SI = 0.67.
- The results of the Langelier Saturation Index proved that the most water treatment processes used at the studied boiler feed water station are inefficient in water aggressiveness removal. The LSI change from −0.34 for raw water to −0.83 in deionized water. With additional aeration, LSI increased from the lowest level up to 0, which made water stable. Extra deaeration sustained this value and kept the water from being aggressive.
- The Ryznar Stability Index (RI) calculation formula is based on the same parameters as LSI. Its calculation showed that experimental water in the whole purification system was aggressive, from RI = 8.24 for raw water to additional aeration with RI of about 7. The last process, deaeration, allowed RI to decrease and reach the not-corrosive-standard of about 5.0.
- An important indicator of water corrosivity and aggressiveness is the change in water colour ΔB. It is calculated with a developed formula that includes many cations and anions. The higher water colour was noticed for primary aeration: about 5.0 mg Pt/dm3. Sand filtration helped in its decrease from 2.1 to 0.0 mg Pt/dm3 after extra aeration and degassing.
Author Contributions
Funding
Conflicts of Interest
References
- Odum, B.; Odum, H. Concepts and methods of ecological engineering. Ecol. Eng. 2003, 20, 339–361. [Google Scholar] [CrossRef]
- Awual, R.; Hasan, M.; Islam, A.; Rahman, M.M.; Asirib, A.; Khalequed, A.; Sheikhe, C. Introducing an amine functionalized novel conjugate material for toxic nitrite detection and adsorption from wastewater. J. Clean. Prod. 2019, 228, 778–785. [Google Scholar] [CrossRef]
- Awual, R. New type mesoporous conjugate material for selective optical copper(II) ions monitoring & removal from polluted waters. Chem. Eng. J. 2017, 307, 85–94. [Google Scholar] [CrossRef]
- Loewenthal, R.E.; Morrison, I.; Wentzel, M.C. Control of corrosion and aggressiveness in drinking water systems. Water Sci. Technol. 2004, 49, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, J.; Stanisławiak, U.R.; Swietlik, J.; Olejnik, A.; Sroka, M.J. Corrosion in a distribution system: Steady water and its composition. Water Res. 2010, 44, 1863–1872. [Google Scholar] [CrossRef]
- Ahmad, J.; Purbolaksono, J.; Beng, L.C. Thermal fatigue and corrosion fatigue in heat recovery area wall side tubes. Eng. Fail. Anal. 2010, 17, 224–343. [Google Scholar] [CrossRef]
- Awual, R. Ring size dependent crown ether based mesoporous adsorbent for high cesium adsorption from wastewater. Chem. Eng. J. 2016, 303, 539–546. [Google Scholar] [CrossRef]
- Awual, R.; Alharthi, N.H.; Hasan, M.; Karim, M.R.; Islam, A.; Znad, H.; Hossain, M.A.; Halim, E.; Rahman, M.M.; Khaleque, A. Inorganic-organic based novel nano-conjugate material for effective cobalt(II) ions capturing from wastewater. Chem. Eng. J. 2017, 324, 130–139. [Google Scholar] [CrossRef]
- Głuszko, M. Atmospheric corrosion of steel structures. Protection methods according to EU requirements. Conference Proceedings New Chemical Products for Mine Industry; Scientific Conference New Chemical Products for Mine Industry: Ustron, Poland, 2007. [Google Scholar]
- Skoczko, I.; Szatylowicz, E. The analysis of physico-chemical properties of two unknown filter materials. J. Ecol. Eng. 2016, 17, 148–154. [Google Scholar] [CrossRef]
- Awual, R. Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem. Eng. J. 2017, 307, 356–465. [Google Scholar] [CrossRef]
- Imran, S.A.; Dietz, J.D.; Mutoti, G.; Taylor, J.S.; Randall, A.A. Modified Larsons Ratio Incorporating Temperature, Water Age, and Electroneutrality Effects on Red Water Release. J. Environ. Eng. 2005, 131, 1514–1520. [Google Scholar] [CrossRef]
- Skoczko, I.; Piekutin, J.; Ignatowicz, K. Efficiency of manganese removal from water in selected filter beds. Desalin. Water Treat. 2016, 57, 1611–1619. [Google Scholar] [CrossRef]
- Awual, R. A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Schock, M.R.; Lytle, D.A. Internal Corrosion and Deposition Control. In Water Quality and Treatment: A Handbook of Community Water Supplies, 6th ed.; AWWA, Edzwald, J.K., Eds.; McGraw-Hill, Inc.: New York, NY, USA, 2010; Chapter 20; pp. 20.1–20.68. [Google Scholar]
- Skoczko, I.; Piekutin, J.; Roszczenko, A. Iron and manganese removal from groundwater by filtration on selected masses. Rocznik Ochrona Srodowiska 2015, 17, 1587–1608. [Google Scholar]
- Awual, R. Novel conjugated hybrid material for efficient lead(II) capturing from contaminated wastewater. Mater. Sci. Eng. C 2019, 101, 686–695. [Google Scholar] [CrossRef]
- Konieczny, K.; Rajca, M.; Bodzek, M.; Kwiecińska, M. Water treatment using hybrid method of coagulation and low pressure membrane filtration. Environ. Prot. Eng. 2009, 35, 5–23. [Google Scholar]
- Siwiec, T.; Michel, M.M.; Reczek, L. Effect of aeration on the change of corrosive aggressiveness of underground water in relation to concrete and steel. Acta Sci. Pol. Archit. 2016, 15, 95–105. [Google Scholar]
- Skoczko, I. Experience with the implementation and design of boiler water treatment station. Annu. Set Environ. Prot. 2011, 13, 657–671. [Google Scholar]
- Frayne, C. The Metro Handbook of Water Treatment for HVAC Systems; The Metro Group, Servicing Water Systems with Environmental Care: New York, NY, USA, 2011. [Google Scholar]
- American Water Works Association. AWWA J100-10 (R13) Risk and Resilience Management of Water and Wastewater Systems; AWWA Catalog No: 40100; AWWA: Denver, CO, USA, 2010. [Google Scholar]
- Anielak, A.M.; Arendacz, M. Iron and manganese removal efficiency using zeolites. Annu. Set Environ. Prot. 2007, 9, 1034–1043. [Google Scholar]
- Sobczyk, M. Pure oxygen usage for groundwater treatment. Environ. Prot. 2001, 4, 466–472. [Google Scholar]
- Shannon, K. Eco-engineering for water: From soft to hard and back. Resilience Ecology and Urban Design; Springer: Dordrecht, The Netherlands, 2012; Volume 3, pp. 163–182. [Google Scholar]
- Sozański, M.M.; Dymaczewski, Z.; Jeż-Walkowiak, J. Development directions and new technologies of water purification. In Conference Procedeengs of IV Scientific Conference, Development Directions of Water Distributions Systems; Seidel-Przywecka, M., Ed.; Seidel-Przywecki Publisher: Warszawa, Poland, 2007; pp. 68–124. [Google Scholar]
- Lee, S.H.J.; Themelis, N.J.; Castaldi, M.J. High-Temperature Corrosion in Waste-to-Energy Boilers. J. Therm. Spray Technol. 2006, 16, 104–110. [Google Scholar] [CrossRef]
- Sarin, P. Corrosion Control Optimization Using Lead Pipe Loops. In Proceedings of the AWWA Water Quality Technology Conference, Miami Beach, FL, USA, 13–17 June 2004; Volume 130, pp. 364–373. [Google Scholar]
- Näykki, T.; Jalukse, L.; Helm, I.; Leito, I. Dissolved Oxygen Concentration Interlaboratory Comparison: What Can We Learn? Water 2013, 5, 420–442. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, K. Failure analysis of boiler cold and hot reheater tubes. Eng. Fail. Anal. 2007, 14, 620–625. [Google Scholar] [CrossRef]
- Soldatov, V.S.; Polus, Z.; Pawłowska, M.; Mączka, I.; Shunkevich, A.; Kasandrovich, E.; Polikarpova, A. A Strong Acid Nonwoven Filtering Medium for Deep Air Purification. Fibres Text. East. Eur. 2004, 4, 56–61. [Google Scholar]
- Ofman, P.; Skoczko, I. PAH removal effectiveness comparison from hydraulic fracturing model wastewater in SBR reactors with granular and flocked activated sludge. Desalin. Water Treat. 2018, 134, 41–51. [Google Scholar] [CrossRef]
- Čokorilo Ilić, M.; Mladenović, A.; Ćuk, M.; Jemcov, I. The Importance of Detailed Groundwater Monitoring for Underground Structure in Karst (Case Study: HPP Pirot, Southeastern Serbia). Water 2019, 11, 603. [Google Scholar] [CrossRef]
- Awual, R. Innovative composite material for efficient and highly selective Pb(II) ion capturing from wastewater. J. Mol. Liq. 2019, 284, 502–510. [Google Scholar] [CrossRef]
- Skoczko, I.; Szatylowicz, E. Studies on the efficiency of grundwater treatment process with adsorption on activated alumina. J. Ecol. Eng. 2017, 18, 211–218. [Google Scholar] [CrossRef]





| Water Parameter | Raw Water | Pre-Aeration | Sand Filter | Cation Exchange | Anion Exchange | Extra Aeration | Extra Deaerator |
|---|---|---|---|---|---|---|---|
| pH | 7.15 | 7.62 | 7.47 | 6.58 | 8.65 | 8.45 | 8.35 |
| Dissolved O2 [mg/dm3] | 0.5 | 3.5 | 2.6 | 0.5 | 0.5 | 3.2 | 0.0 |
| Temp. [0C] | 11.7 | 13.6 | 15.1 | 15.4 | 15.2 | 15.1 | 97.9 |
| Conductivity [mS/dm3] | 0.75 | 0.73 | 0.56 | 0.13 | 0.07 | 0.07 | 0.07 |
| Cl−[mg/dm3] | 80 | 80 | 80 | 80 | 12 | 12 | 12 |
| SO2−4 [mg/dm3] | 176 | 176 | 112 | 112 | 18 | 18 | 18 |
| Total Hardness [mgCaCO3/dm3] | 450 | 450 | 380 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ca [mg/dm3] | 132 | 132 | 115 | 0.0 | 0.0 | 0.0 | 0.0 |
| CO2 (free) [mg/dm3] | 91 | 65 | 56 | 47 | 6 | 0.0 | 0.0 |
| CO2 (bicarb.) [mg/dm3] | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CO2 (carb.) [mg/dm3] | 231 | 185 | 176 | 155 | 25 | 25 | 25 |
| CO2 (aggress.) [mg/dm3] | 2.5 | 1.9 | 1.9 | 2.0 | 0.2 | 0.0 | 0.0 |
| Tot.Diss.Solids [g/dm3] | 32 | 32 | 13 | 2.5 | 0.4 | 0.4 | 0.4 |
| Tot. alk. [mval/dm3] | 3.4 | 4.5 | 4.1 | 3.5 | 5.5 | 5.5 | 5.5 |
| HRT [day] | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Na+ [mg/dm3] | 170 | 98 | 98 | 98 | 98 | 98 | 98 |
| ΔB [mg Pt/dm3] | 3.1 | 4.9 | 2.1 | 0.3 | 0.3 | 0.0 | 0.0 |
| LSI | RI | LI | SI | ||
|---|---|---|---|---|---|
| Raw water | −0.34 | 8.24 | 0.22 | 1.04 | |
| Pre-aerated water | −0.12 | 7.23 | 0.25 | 0.93 | |
| Filtrated water | −0.23 | 7.57 | 0.36 | 0.85 | |
| Deionized water (cation and anion exchange) | −0.83 | 9.03 | 0.28 | 0.81 | |
| Secondary aeration | 0 | 7.05 | 0.23 | 0.75 | |
| Extra deaeration | 0 | 5.89 | 0.21 | 0.67 |
| Values of Water Aggressiveness and Corrosivity Indexes | ||||
|---|---|---|---|---|
| Index | LSI | RI | LI | SI |
| Corrosive/aggressive water | JL < 0 | JR > 7 | IL >> 1.2 | IL >> 1.2 |
| Stable water | JL = 0 | JR = 6–7 | 1.0 < IL<1.2 | 1.0 < IL < 1.2 |
| Not corrosive/not aggressive water | JL > 0 | JR < 5 | IL < 0.8 | IL < 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoczko, I.; Szatyłowicz, E. Treatment Method Assessment of the Impact on the Corrosivity and Aggressiveness for the Boiler Feed Water. Water 2019, 11, 1965. https://doi.org/10.3390/w11101965
Skoczko I, Szatyłowicz E. Treatment Method Assessment of the Impact on the Corrosivity and Aggressiveness for the Boiler Feed Water. Water. 2019; 11(10):1965. https://doi.org/10.3390/w11101965
Chicago/Turabian StyleSkoczko, Iwona, and Ewa Szatyłowicz. 2019. "Treatment Method Assessment of the Impact on the Corrosivity and Aggressiveness for the Boiler Feed Water" Water 11, no. 10: 1965. https://doi.org/10.3390/w11101965
APA StyleSkoczko, I., & Szatyłowicz, E. (2019). Treatment Method Assessment of the Impact on the Corrosivity and Aggressiveness for the Boiler Feed Water. Water, 11(10), 1965. https://doi.org/10.3390/w11101965