Advanced Oxidation Based Treatment of Soil Wash Water Contaminated with Sulfolane
Abstract
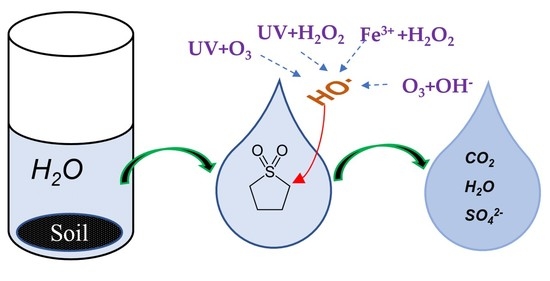
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Procedure
2.2.1. Soil Washing Standalone
2.2.2. Advanced Oxidation Process Treatment of Soil Washing Water
- For UV/H2O2 experiments, a Luzchem photoreactor equipped with 10 germicidal lamps (8 Watt LZC-UVC, Luzchem), was used. The photoreactor has a 30 cm wide, 30 cm deep, and 22 cm high chamber as shown in Figure S1. The chamber is made of aluminum alloy (Al 5052-H32), which can serve as good reflector. There are five lamps on each side of the chamber. The number of photons received by 100 mL solution in the quartz beaker per unit time was measured to be 5.3 × 1017 photon/s through ferrioxalate actinometry [19]. Four different H2O2 dosages were applied to each diluted sample.
- For UV/O3 experiments, ozone was generated through an O3 generator (A2Z 5-G LAB, A2Z O3 systems Inc.), which was introduced into the solution through a glass diffuser. Samples for each dilution were treated with four different ozone flow rates (1.9, 2.9, 3.7, and 4.3 g/h). The ozone flow rates were calculated based on ozone concentration in ozone generator outlet and the gas volumetric flow rate.
- For alkaline ozonation, sodium hydroxide was gradually added into the solution to adjust the pH from 10 to 13 before exposure to ozone. Ozone flow rate was kept at 3.7 g/h.
- For neutral Fenton experiments, FeEDTA and H2O2 were introduced into the solution at a molar ratio of 1:10. The FeEDTA concentrations were 0.4 mM (147 mg/L), 2 mM (734 mg/L), and 10 mM (3670 mg/L). A total of 100 mg of sodium thiosulfate was used to quench the reaction.
2.2.3. Extraction and GC Analysis
3. Results and Discussions
3.1. Soil Washing
3.2. AOP as a Post Soil Washing Treatment
3.2.1. UV/H2O2
3.2.2. UV/O3
3.2.3. Alkaline Ozonation
3.2.4. Neutral Fenton
4. Conclusions
- UVC irradiation combined with appropriate H2O2 concentration can remove more than 99% of sulfolane within 1 h. The suitable H2O2 concentration was determined to be 200 mg/L in undiluted sample, leading to a k value of 0.06 min−1.UVC assisted ozonation can effectively degrade sulfolane within 30 min. The suitable ozone flow rate was determined to be 3.7 g/h.
- Ozonation with higher pH can increased the sulfolane degradation in soil wash water.
- Neutral Fenton reagents perform poorly in sulfolane removal. Less than 50% of sulfolane removal were observed within 1 h.
- Dilution can increase sulfolane degradation kinetics for all AOP treatments. Neutral Fenton is least sensitive to dilution effect, whereas alkaline ozonation is highly sensitive to dilution.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Mousset, E.; Huguenot, D.; van Hullebusch, E.D.; Oturan, N.; Guibaud, G.; Esposito, G.; Oturan, M.A. Impact of electrochemical treatment of soil washing solution on PAH degradation efficiency and soil respirometry. Environ. Pollut. 2016, 211, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kang, H.; Do, W. Application of nonionic surfactant-enhanced in situ flushing to a diesel contaminated site. Water Res. 2005, 39, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Baziar, M.; Mehrasebi, M.R.; Assadi, A.; Fazli, M.M.; Maroosi, M.; Rahimi, F. Efficiency of non-ionic surfactants-EDTA for treating TPH and heavy metals from contaminated soil. J. Environ. Health Sci. Eng. 2013, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-H.; Zhao, L.; Lin, Z.-R.; Dong, Y.-H. Soil washing in combination with homogeneous Fenton-like oxidation for the removal of 2,4,4′-trichlorodiphenyl from soil contaminated with capacitor oil. Environ. Sci. Pollut. Res. 2016, 23, 7890–7898. [Google Scholar] [CrossRef]
- Gordon, C.J.; Dyer, R.S.; Long, M.D.; Fehlner, K.S. Effect of sulfolane on behavioral and autonomic thermoregulation in the rat. J. Toxicol. Environ. Health 1985, 16, 461–468. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Sun, M.L.; Li, Z.S.; Yang, Z.C.; Zhang, T.B.; Heng, Z.C.; Xiao, B.L.; Li, Q.Y.; Peng, Q.Y.; Dong, Y.H. [An investigation of the maximum allowable concentration of sulfolane in surface water]. Hua Xi Yi Ke Da Xue Xue Bao 1987, 18, 376–380. [Google Scholar]
- Yu, L.; Mehrabani-Zeinabad, M.; Achari, G.; Langford, C.H. Application of UV based advanced oxidation to treat sulfolane in an aqueous medium. Chemosphere 2016, 160, 155–161. [Google Scholar] [CrossRef]
- Izadifard, M.; Achari, G.; Langford, C.H. Degradation of sulfolane using activated persulfate with UV and UV-Ozone. Water Res. 2017, 125, 325–331. [Google Scholar] [CrossRef]
- Khan, M.F.; Yu, L.; Achari, G.; Tay, J.H. Degradation of sulfolane in aqueous media by integrating activated sludge and advanced oxidation process. Chemosphere 2019, 222, 1–8. [Google Scholar] [CrossRef]
- Khan, M.F.; Yu, L.; Tay, J.H.; Achari, G. Coaggregation of bacterial communities in aerobic granulation and its application on the biodegradation of sulfolane. J. Hazard. Mater. 2019, 377, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Saint-Fort, R. Sulfolane attenuation by surface and subsurface soil matrices. J. Environ. Sci. Health A 2006, 41, 1211–1231. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, K.; van Hullebusch, E.D.; Cassir, M.; Bermond, A. Application of advanced oxidation processes for TNT removal: A review. J. Hazard. Mater. 2010, 178, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Rosas, J.M.; Vicente, F.; Santos, A.; Romero, A. Soil remediation using soil washing followed by Fenton oxidation. Chem. Eng. J. 2013, 220, 125–132. [Google Scholar] [CrossRef]
- Mehrabani-Zeinabad, M.; Yu, L.; Achari, G.; Langford, C.H. Mineralisation of sulfolane by UV/O3/H2O2 in a tubular reactor. J. Environ. Eng. Sci. 2016, 11, 44–51. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Palanisami, T.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Ex-Situ Remediation Technologies for Environmental Pollutants: A Critical Perspective. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Reviews of Environmental Contamination and Toxicology; Springer: Cham, Germany, 2016; Volume 236, pp. 117–192. ISBN 978-3-319-20013-2. [Google Scholar]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Goldstein, S.; Rabani, J. The ferrioxalate and iodide–iodate actinometers in the UV region. J. Photochem. Photobiol. A Chem. 2008, 193, 50–55. [Google Scholar] [CrossRef]
- Luther, S.M.; Dudas, M.J.; Fedorak, P.M. Sorption of sulfolane and diisopropanolamine by soils, clays and aquifer materials. J. Contam. Hydrol. 1998, 32, 159–176. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution 1. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; The American Society of Agronomy and Academic Press: New York, USA, 1986; pp. 425–442. [Google Scholar]
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J. Hazard. Mater. 2016, 306, 149–174. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Zanta, C.L.P.S.; Martínez-Huitle, C.A. Degradation of 2-hydroxybenzoic acid by advanced oxidation processes. Braz. J. Chem. Eng. 2009, 26, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Watts Richard, J.; Teel Amy, L. Chemistry of Modified Fenton’s Reagent (Catalyzed H2 O2 Propagations–CHP) for In Situ Soil and Groundwater Remediation. J. Environ. Eng. 2005, 131, 612–622. [Google Scholar] [CrossRef]
- Christensen, H.; Sehested, K.; Corfitzen, H. Reactions of hydroxyl radicals with hydrogen peroxide at ambient and elevated temperatures. J. Phys. Chem. 1982, 86, 1588–1590. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photochemical oxidation of reactive azo dye with UV–H2O2 process. Dye. Pigment. 2004, 62, 269–275. [Google Scholar] [CrossRef]
- Forni, L.; Bahnemann, D.; Hart, E.J. Mechanism of the hydroxide ion-initiated decomposition of ozone in aqueous solution. J. Phys. Chem. 1982, 86, 255–259. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water: Rate of initiation by hydroxide ions and hydrogen peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- De Luca, A.; Dantas, R.F.; Esplugas, S. Assessment of iron chelates efficiency for photo-Fenton at neutral pH. Water Res. 2014, 61, 232–242. [Google Scholar] [CrossRef]
- Yu, L.; Achari, G.; Langford, C.H.; Keir, I. A feasibility study on sulfolane degradation in groundwater using neutral fenton catalysts. In Proceedings of the CSCE 2016, London, ON, Canada, 1–4 June 2016. [Google Scholar]








| Experiment No. | Soil/Water Ratio | Extraction Time per Cycle | No of Cycle |
|---|---|---|---|
| SW1 | 1:2 | 30 min | 1 |
| SW2 | 1:2 | 60 min | 1 |
| SW3 | 1:2 | 90 min | 1 |
| SW4 | 1:1 | 30 min | 1 |
| SW5 | 1:2 | 30 min | 1 |
| SW6 | 1:3 | 30 min | 1 |
| SW7 | 1:2 | 30 min | 3 |
| Type of AOP | Dilution Factor | [Sulfolane] (mg/L) | Photon Flux * (photon/s) | [H2O2] (mg/L) | O3 (g/h) | [FeEDTA] (mg/L) | pH |
|---|---|---|---|---|---|---|---|
| UVC/H2O2 | 1 | 100 | 5.3 × 1017 | 100 | - | - | 7 |
| 200 | |||||||
| 500 | |||||||
| 1000 | |||||||
| 5 | 20 | 5.3 × 1017 | 20 | - | - | 7 | |
| 40 | |||||||
| 100 | |||||||
| 200 | |||||||
| 500 | 0.2 | 5.3 × 1017 | 5 | - | - | 7 | |
| 20 | |||||||
| 40 | |||||||
| 100 | |||||||
| UVC/O3 | 1 | 100 | 5.3 × 1017 | - | 1.9 | - | 7 |
| 2.9 | |||||||
| 3.7 | |||||||
| 4.3 | |||||||
| 5 | 20 | 5.3 × 1017 | - | 1.9 | - | 7 | |
| 2.9 | |||||||
| 3.7 | |||||||
| 4.3 | |||||||
| 500 | 0.2 | 5.3 × 1017 | - | 1.9 | - | 7 | |
| 2.9 | |||||||
| 3.7 | |||||||
| 4.3 | |||||||
| Alkaline O3 | 1 | 100 | - | - | 3.7 | - | 10 |
| 11 | |||||||
| 12 | |||||||
| 13 | |||||||
| 5 | 20 | - | - | 3.7 | - | 10 | |
| 11 | |||||||
| 12 | |||||||
| 13 | |||||||
| Neutral Fenton | 1 | 100 | - | 3400 | - | 3670 | 7 |
| 680 | 734 | ||||||
| 136 | 147 | ||||||
| 5 | 20 | - | 3400 | - | 3670 | 7 | |
| 680 | 734 | ||||||
| 136 | 147 | ||||||
| 500 | 0.2 | - | 3400 | - | 3670 | 7 | |
| 680 | 734 | ||||||
| 136 | 147 |
| Parameters | GC-FID | GC-MS |
|---|---|---|
| GC column | ZB 5MSI (Phenomenex) | ZB 5MSI (Phenomenex) |
| Carrier gas type | helium | helium |
| Carrier gas program | constant pressure at 250 kPa | constant flow rate at 1.07 mL/min |
| Injection temperature | 165 °C | 250 °C |
| Injection volume | 1.00 µL | 1.00 µL |
| Mode of injection | splitless | splitless |
| Oven program | 90 °C hold for 2 min; 10 °C/min to 175 °C; 175 °C hold for 3 min. | 90 °C hold for 2 min; 10 °C/min to 160 °C; 20 °C/min to 280 °C; 280 °C hold for 2 min. |
| FID temperature | 330 °C | \ |
| Mode of MS | \ | SIM |
| m/z | \ | 41, 56, and 120 |
| [H2O2] mg/L | Photon Energy Required to Remove 90% of Sulfolane from the Wash Water (kWh/m3) | ||
|---|---|---|---|
| Undiluted | 5× Dilution | 500× Dilution | |
| 5 | / | / | 286.39 |
| 20 | / | 186.45 | 171.71 |
| 40 | / | 103.96 | 210.85 |
| 100 | 6.52 | 29.26 | 254.99 |
| 200 | 2.54 | 12.58 | / |
| 500 | 2.02 | / | / |
| 1000 | 2.50 | / | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, M.; Yu, L.; Garcia, C.; Achari, G. Advanced Oxidation Based Treatment of Soil Wash Water Contaminated with Sulfolane. Water 2019, 11, 2152. https://doi.org/10.3390/w11102152
Brandão M, Yu L, Garcia C, Achari G. Advanced Oxidation Based Treatment of Soil Wash Water Contaminated with Sulfolane. Water. 2019; 11(10):2152. https://doi.org/10.3390/w11102152
Chicago/Turabian StyleBrandão, Mariana, Linlong Yu, Carlos Garcia, and Gopal Achari. 2019. "Advanced Oxidation Based Treatment of Soil Wash Water Contaminated with Sulfolane" Water 11, no. 10: 2152. https://doi.org/10.3390/w11102152
APA StyleBrandão, M., Yu, L., Garcia, C., & Achari, G. (2019). Advanced Oxidation Based Treatment of Soil Wash Water Contaminated with Sulfolane. Water, 11(10), 2152. https://doi.org/10.3390/w11102152