Short-Term Responses of Aquatic and Terrestrial Biodiversity to Riparian Restoration Measures Designed to Control the Invasive Arundo donax L.
Abstract
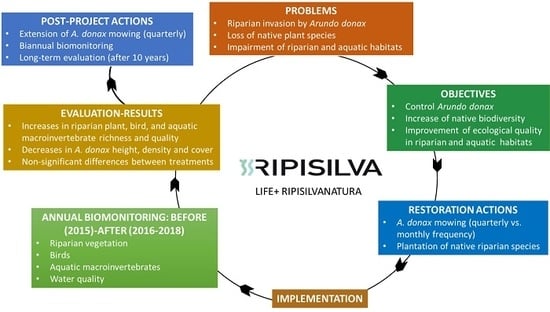
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Restoration Actions
2.3. Biomonitoring and Ecological Indicators
2.4. Data Analysis
3. Results
4. Discussion
4.1. Riparian Vegetation
4.2. Aquatic Macroinvertebrates
4.3. Birds
4.4. Management Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef] [PubMed]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Tickner, D.P.; Angold, P.G.; Gurnell, A.M.; Mountford, J.O. Riparian plant invasions: Hydrogeomorphological control and ecological impacts. Prog. Phys. Geogr. 2001, 25, 22–52. [Google Scholar] [CrossRef]
- Richardson, D.M.; Van Wilgen, B.W. Invasive alien plants in South Africa: How well do we understand the ecological impacts? Working for water. S. Afr. J. Sci. 2004, 100, 45–52. [Google Scholar]
- Saunders, D.L.; Meeuwig, J.J.; Vincent, A.C.J. Freshwater protected areas: Strategies for conservation. Conserv. Biol. 2002, 16, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.M.; Dudley, T.L.; Saltonstall, K. Ecology and impacts of the large-statured invasive grasses Arundo donax and Phragmites australis in North America. Invasive Plant Sci. Manag. 2010, 3, 489–494. [Google Scholar] [CrossRef]
- Herrera, A.M.; Dudley, T.L. Reduction of riparian arthropod abundance and diversity as a consequence of giant reed (Arundo donax) invasion. Biol. Invasions 2003, 5, 167–177. [Google Scholar] [CrossRef]
- Coffman, G.C. Factors Influencing Invasion of Giant Reed (Arundo donax) in Riparian Ecosystems of Mediterranean-Type Climate Regions. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2007. [Google Scholar]
- Kisner, D.A. The Effect of Giant Reed (Arundo donax) on the Southern California Riparian Bird Community. Master’s Thesis, San Diego State University, San Diego, CA, USA, 2004. [Google Scholar]
- Hardion, L.; Verlaque, R.; Saltonstall, K.; Leriche, A.; Vila, B. Origin of the invasive Arundo donax (Poaceae): A trans-Asian expedition in herbaria. Ann. Bot. 2014, 114, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2004. [Google Scholar]
- Dudley, T.L. Arundo donax L. In Invasive Plants of California’s Wildlands; Bossard, C.C., Randall, J.M., Hoshovsky, M.C., Eds.; University of California Press: Berkeley/Los Angeles, CA, USA, 2000; pp. 53–58. [Google Scholar]
- Coffman, G.C.; Ambrose, R.F.; Rundel, P.W. Wildfire promotes dominance of invasive giant reed (Arundo donax) in riparian ecosystems. Biol. Invasions 2010, 12, 2723–2734. [Google Scholar] [CrossRef] [Green Version]
- Bruno, D.; Belmar, O.; Sánchez-Fernández, D.; Velasco, J. Environmental determinants of woody and herbaceous riparian vegetation patterns in a semi-arid Mediterranean basin. Hydrobiologia 2014, 730, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Bell, G.P. Ecology and management of Arundo donax and approaches to riparian habitat restoration in southern California. In Plant Invasions: Studies from North America and Europe; Brock, J.H., Wade, M., Pysek, P., Green, D., Eds.; Backhuys Publishers: Kerkwerve, The Netherlands, 1997; pp. 110–113. [Google Scholar]
- Perdue, R.E. Arundo donax—Source of musical reeds and industrial cellulose. Econ. Bot. 1958, 12, 368–404. [Google Scholar] [CrossRef]
- Quinn, L.D.; Holt, J.S. Ecological correlates of invasion by Arundo donax in three southern California riparian habitats. Biol. Invasions 2008, 10, 591–601. [Google Scholar] [CrossRef]
- Mann, J.J.; Barney, J.N.; Kyser, G.B.; Di Tomaso, J.M. Miscanthus × giganteus and Arundo donax shoot and rhizome tolerance of extreme moisture stress. GCB Bioenergy 2013, 5, 693–700. [Google Scholar] [CrossRef]
- Guthrie, G. Impacts of the Invasive Reed Arundo donax on Biodiversity at the Community-Ecosystem Level. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2007. [Google Scholar]
- Milton, S.J. Grasses as invasive alien plants in South Africa. S. Afr. J. Sci. 2004, 100, 69–75. [Google Scholar]
- Maceda-Veiga, A.; Basas, H.; Lanzaco, G.; Sala, M.; de Sostoa, A.; Serra, A. Impacts of the invader giant reed (Arundo donax) on riparian habitats and ground arthropod communities. Biol. Invasions 2016, 18, 731–749. [Google Scholar] [CrossRef]
- Quinn, L.D.; Rauterkus, M.A.; Holt, J.S. Effects of nitrogen enrichment and competition on growth and spread of giant reed (Arundo donax). Weed Sci. 2007, 55, 319–326. [Google Scholar] [CrossRef]
- Gregory, S.V.; Swanson, F.J.; McKee, W.A.; Cummins, K.W. An ecosystem perspective of riparian zones. BioScience 1991, 41, 540–551. [Google Scholar] [CrossRef]
- Sabater, F.; Butturini, A.; Martí, E.; Muñoz, I.; Romaní, A.; Wray, J.; Sabater, S. Effects of riparian vegetation removal on nutrient retention in a Mediterranean stream. J. N. Am. Benthol. Soc. 2000, 19, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Naiman, R.J.; Decamps, H.; McClain, M.E. Riparia: Ecology, Conservation, and Management of Streamside Communities; Elsevier: New York, NY, USA, 2005. [Google Scholar]
- Giessow, J.; Casanova, J.; Leclerc, R.; MacArthur, R.; Fleming, G.; Giessow, J. Arundo Donax (Giant Reed): Distribution and Impact Report; State Water Resources Control Board; California Invasive Plant Council (Cal-IPC): 2011. pp. 1–240. Available online: https://www.cal-ipc.org/wp-content/uploads/2017/11/Arundo_Distribution_Impact_Report_Cal-IPC_March-2011_small.pdf (accessed on 27 November 2018).
- Chamier, J.; Schachtschneider, K.; Le Maitre, D.C.; Ashton, P.J.; Van Wilgen, B.W. Impacts of invasive alien plants on water quality, with particular emphasis on South Africa. Water SA 2012, 38, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Frandsen, P.; Jackson, N. Impact of Arundo donax on flood control and endangered species. In Arundo donax Workshop Proceedings; Jackson, N.E., Frandsen, S., Duthoit, S., Eds.; Team Arundo and California Exotic Pest Plant Council: Pismo Beach, CA, USA, 1994; pp. 13–16. [Google Scholar]
- Iverson, M. The impact of Arundo donax on water resources. In Arundo donax Workshop Proceedings; Jackson, N.E., Frandsen, S., Duthoit, S., Eds.; Team Arundo and California Exotic Pest Plant Council: Pismo Beach, CA, USA, 1994; pp. 19–26. [Google Scholar]
- Vilà, M.; Hulme, P.E. Impact of Biological Invasions on Ecosystem Services; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Deltoro-Torró, V.; Jimenez-Ruiz, J.; Vilan-Fragueiro, X.M. Bases Para El Manejo Y Control de Arundo donax L. (Caña común); Colección Manuales Técnicos de Biodiversidad, 4. Conselleria d’Infraestructures; Territori i Medi Ambient, Generalitat Valenciana: Valencia, Spain, 2012. [Google Scholar]
- Spencer, D.F.; Colby, L.; Norris, G.R. An evaluation of flooding risks associated with giant reed (Arundo donax). J. Freshw. Ecol. 2013, 28, 397–409. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Moran, P. Host range of Tetramesa romana Walker (Hymenoptera: Eurytomidae), a potential biological control of giant reed, Arundo donax L. in North America. Biol. Control 2009, 49, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Miles, D.H.; Tunsuwan, K.; Chittawong, V.; Kokpol, U.; Choudhary, M.I.; Clardy, J. Boll weevil antifeedants from Arundo donax. Phytochemistry 1993, 34, 1277–1279. [Google Scholar] [CrossRef]
- Mackenzie, A. Giant reed. In The Weed Workers’ Handbook; Harrington, C., Hayes, A., Eds.; The Watershed Project and California Invasive Plant Council: California, CA, USA, 2014; pp. 92–93. [Google Scholar]
- Going, B.M.; Dudley, T.L. Invasive riparian plant litter alters aquatic insect growth. Biol. Invasions 2008, 10, 1041–1051. [Google Scholar] [CrossRef]
- Bruno, D.; Zapata, V.; Conesa, A.; Guareschi, S.; Picazo, F.; Dettori, E.; Millán, A.; Robledano, F.; Velasco, J. LIFE+ RIPISILVANATURA: Biomonitoring and Short-Term Assessment of Restoration Measures to Control Invasive Alien Species in the Segura River (Spain); I.S. Rivers: Lyon, France, 2018. [Google Scholar]
- Godinho, C.; Rabaça, J.; Segurado, P. Breeding bird assemblages in riparian galleries of the Guadiana River basin (Portugal): The effect of spatial structure and habitat variables. Ecol. Res. 2010, 25, 283–294. [Google Scholar] [CrossRef]
- Machtans, C.S.; Villard, M.A.; Hannon, S.J. Use of riparian buffer strips as movement corridors by forest birds. Conserv. Biol. 1996, 10, 1366–1379. [Google Scholar] [CrossRef]
- Stevens, L.E.; Brown, B.T.; Simpson, J.M.; Johnson, R.R. The Importance of Riparian Habitat to Migrating Birds. In Importance, Preservation, and Management of Riparian Habitat; Johnson, R.R., Jones, D.A., Eds.; USDA Forest Service: Washington, DC, USA, 1977; pp. 156–164. [Google Scholar]
- Paracuellos, M. Análisis comparativo entre las comunidades de Passeriformes de cañaverales y carrizales en el sureste ibérico. Ardeola 1997, 44, 105–108. [Google Scholar]
- Corbacho, C.; Sánchez, J.M.; Costillo, E. Patterns of structural complexity and human disturbance of riparian vegetation in agricultural landscapes of a Mediterranean area. Agric. Ecosyst. Environ. 2003, 95, 495–507. [Google Scholar] [CrossRef]
- Puértolas, L.; Damásio, J.; Barata, C.; Soares, A.M.; Prat, N. Evaluation of side-effects of glyphosate mediated control of giant reed (Arundo donax) on the structure and function of a nearby Mediterranean river ecosystem. Environ. Res. 2010, 110, 556–564. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, J.; Santín-Montanyá, M.I. An approach to the integrated management of exotic invasive weeds in riparian zones. In Riparian Zones: Characteristics, Management Practices, and Ecological Impacts; Pokrovsky, O.S., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 99–124. [Google Scholar]
- San Martín, C.; Gourlie, J.A.; Barroso, J. Control of Volunteer Giant Reed (Arundo donax). Invasive Plant Sci. Manag. 2019, 12, 43–50. [Google Scholar] [CrossRef]
- Thornby, D.; Spencer, D.; Hanan, J.; Sher, A. L-DONAX, a growth model of the invasive weed species, Arundo donax L. Aquat. Bot. 2007, 87, 275–284. [Google Scholar] [CrossRef]
- Neeson, T.M.; Ferris, M.C.; Diebel, M.W.; Doran, P.J.; O’Hanley, J.R.; McIntyre, P.B. Enhancing ecosystem restoration efficiency through spatial and temporal coordination. Proc. Natl. Acad. Sci. USA 2015, 112, 6236–6241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadmeadow, S.; Nisbet, T.R. The effects of riparian forest management on the freshwater environment: A literature review of best management practice. Hydrol. Earth Syst. Sci. 2004, 8, 286–305. [Google Scholar] [CrossRef]
- Marczak, L.B.; Sakamaki, T.; Turvey, S.L.; Deguise, I.; Wood, S.L.; Richardson, J.S. Are forested buffers an effective conservation strategy for riparian fauna? An assessment using meta-analysis. Ecol. Appl. 2010, 20, 126–134. [Google Scholar] [CrossRef]
- Hooper, E.; Condit, R.; Legendre, P. Responses of 20 native tree species to reforestation strategies for abandoned farmland in Panama. Ecol. Appl. 2002, 12, 1626–1641. [Google Scholar] [CrossRef]
- Belmar, O.; Bruno, D.; Martínez-Capel, F.; Barquín, J.; Velasco, J. Effects of flow regime alteration on fluvial habitats and riparian quality in a semiarid Mediterranean basin. Ecol. Indic. 2013, 30, 52–64. [Google Scholar] [CrossRef]
- Belmar, O.; Bruno, D.; Guareschi, S.; Mellado-Díaz, A.; Millán, A.; Velasco, J. Functional responses of aquatic macroinvertebrates to flow regulation are shaped by natural flow intermittence in Mediterranean streams. Freshw. Biol. 2019, 64, 1064–1077. [Google Scholar] [CrossRef]
- Bruno, D. Respuestas estructurales y funcionales de las comunidades riparias mediterráneas a los filtros ambientales. Ecosistemas 2016, 25, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Velasco, J.; Robledano, F.; Bruno, D.; Zapata, V.M.; Calvo, J.F.; Millán, A. Acciones preparatorias y de seguimiento para la recuperación de los hábitats riparios y control de especies exóticas en la cuenca del río Segura. In Proceedings of the II Iberian Conference on River Restoration, Pamplona, Spain, 9–11 June 2015; Centro Ibérico de Restauración Fluvial: Zaragoza, Spain; pp. 568–577. [Google Scholar]
- Gonzalez del Tánago, M.; García de Jalón, D. Riparian Quality Index (RQI): A methodology for characterising and assessing the environmental conditions of riparian zones. Limnetica 2011, 30, 235–254. [Google Scholar]
- Sutherland, W.J. Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Buckland, S.T. Point-Transect Surveys for Songbirds: Robust Methodologies. Auk Ornithol. Adv. 2006, 123, 345–357. [Google Scholar]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S. Bird Census Techniques, 2nd ed.; Elsevier: Oxford, UK, 2000. [Google Scholar]
- Jáimez-Cuéllar, P.; Vivas, S.; Bonada, N.; Robles, S.; Mellado, A.; Álvarez, M.; Avilés, J.; Casas, J.; Ortega, M.; Pardo, I.; et al. Protocolo GUADALMED (prece). Limnetica 2002, 21, 187–204. [Google Scholar]
- Alba-Tercedor, J.; Jáimez-Cuéllar, P.; Álvarez, M.; Avilés, J.; Bonada, N.; Casas, J.; Mellado, A.; Ortega, M.; Pardo, I.; Prat, N. Caracterización del estado ecológico de ríos mediterráneos ibéricos mediante el índice IBMWP (antes BMWP’). Limnetica 2002, 21, 175–185. [Google Scholar]
- Sánchez-Fernández, D.; Abellán, P.; Mellado, A.; Velasco, J.; Millán, A. Are water beetles good indicators of biodiversity in Mediterranean aquatic ecosystems? The case of the Segura river basin (SE Spain). Biodivers. Conserv. 2006, 15, 4507–4520. [Google Scholar] [CrossRef]
- Carbonell, J.A.; Gutiérrez-Cánovas, C.; Bruno, D.; Abellán, P.; Velasco, J.; Millán, A. Ecological factors determining the distribution and assemblages of the aquatic Hemiptera (Gerromorpha & Nepomorpha) in the Segura River basin (Spain). Limnetica 2011, 30, 59–70. [Google Scholar]
- Dray, S.; Dufour, A.B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B.; Ellison, S.; Firth, D.; Friendly, M.; et al. Package ‘Car’; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using “lme4”. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S.; Hothorn, M.T. Package ‘Multcomp’. Simultaneous Inference in General Parametric Models; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Wang, Y.; Naumann, U.; Wright, S.T.; Warton, D.I. Mvabund—An R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Barton, K.; Barton, M.K. Package ‘MuMIn’, version 1.18; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.R. Development Core Team. In Nlme: Linear and Nonlinear Mixed Effects Model; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B. Vegan: Community Ecology Package; R Foundation for Statistical Computing: Vienna, Austria, 2007. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 15 December 2017).
- Else, J.A. Post-Flood Establishment of Native Woody Species and an Exotic, Arundo donax, in a Southern California Riparian System. Master’s Thesis, San Diego State University, San Diego, CA, USA, 1996. [Google Scholar]
- Boose, A.B.; Holt, J.S. Environmental effects on asexual reproduction in Arundo donax. Weed Res. 1999, 39, 117–127. [Google Scholar] [CrossRef]
- Sharma, K.P.; Kushwaha, S.P.S.; Gopal, B. A comparative study of stand structure and standing crops of two wetland species, Arundo donax and Phragmites karka, and primary production in Arundo donax with observations on the effect of clipping. Trop. Ecol. 1998, 39, 3–14. [Google Scholar]
- Rossa, B.; Tuffers, A.V.; Naidoo, G.; Von Willert, D.J. Arundo donax L. (Poaceae)—A C3 species with unusually high photosynthetic capacity. Bot. Acta 1998, 111, 216–221. [Google Scholar] [CrossRef]
- Godé, L.; García, E.; Gutierrez, I.; Perearnau, C. La Gestió I Recuperació de La Vegetació de Ribera: Guia Tècnica Per a Actuacions en Riberes; Departament de Medi Ambient i Habitatge, Generalitat de Catalunya: Barcelona, Spain, 2008. [Google Scholar]
- Mota i Freixas. Estudi de noves tècniques per a l’eradicació de l’Arundo donax. Bachelor’s Thesis, Environmental Sciences-Universitat Autònoma de Barcelona, Barcelona, Spain, 2009.
- Racelis, A.E.; Rubio, A.; Vaughan, T.; Goolsby, J.A. Passive restoration potential of riparian areas invaded by giant reed (Arundo donax) in Texas. Ecol. Res. 2012, 30, 103–105. [Google Scholar] [CrossRef]
- González, E.; Sher, A.A.; Tabacchi, E.; Masip, A.; Poulin, M. Restoration of riparian vegetation: A global review of implementation and evaluation approaches in the international, peer-reviewed literature. J. Environ. Manag. 2015, 158, 85–94. [Google Scholar] [CrossRef]
- Buchanan, B.P.; Nagle, G.N.; Walter, M.T. Long-term monitoring and assessment of a stream restoration project in central New York. River Res. Appl. 2014, 30, 245–258. [Google Scholar] [CrossRef]
- Bruno, D.; Belmar, O.; Sánchez-Fernández, D.; Guareschi, S.; Millán, A.; Velasco, J. Responses of Mediterranean aquatic and riparian communities to human pressures at different spatial scales. Ecol. Indic. 2014, 45, 456–464. [Google Scholar] [CrossRef]
- Sabater, S. Alterations of the global water cycle and their effects on river structure, function and services. Freshw. Rev. 2008, 1, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Bunn, S.E.; Davies, P.M.; Kellaway, D.M. Contributions of sugar cane and invasive pasture grass to the aquatic food web of a tropical lowland stream. Mar. Freshw. Res. 1997, 48, 173–179. [Google Scholar] [CrossRef]
- Sampaio, A.; Cortes, R.; Leão, C. Invertebrate and microbial colonisation in native and exotic leaf litter species in a mountain stream. Int. Rev. Hydrobiol. 2001, 86, 527–540. [Google Scholar] [CrossRef]
- Read, M.G.; Barmuta, L.A. Comparisons of benthic communities adjacent to riparian native eucalypt and introduced willow vegetation. Freshw. Biol. 1999, 42, 359–374. [Google Scholar] [CrossRef]
- Naeem, S.; Thompson, L.J.; Lawler, S.P.; Lawton, J.H.; Woodfin, R.M. Declining biodiversity can alter the performance of ecosystems. Nature 1994, 368, 734–737. [Google Scholar] [CrossRef]
- Ródenas, M.A.; Albacete, M. The River Segura: Reclaimed water, recovered river. J. Water Reuse Desalin. 2014, 4, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Marín, J.M.; Zamora-López, A.; Sánchez-Pérez, A.; Torralva, M.; Oliva-Paterna, F.J. Establecimiento de la almeja asiática Corbicula fluminea (Müller, 1774) en la cuenca del río Segura (SE Península Ibérica). Limnetica 2018, 37, 1–7. [Google Scholar]
- Verdú, J.R.; Numa, C.; Galante, E. Atlas Y Libro Rojo de Los Invertebrados Amenazados de España (Especies Vulnerables); Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente; Medio Rural y Marino: Madrid, Spain, 2011. [Google Scholar]
- Vilar, C.M. Respuesta de la biodiversidad ante el cambio global: Caracterización de las comunidades de aves de ribera como indicadores de degradación y restauración. Bachelor’s Thesis, Environmental Sciences-Universidad de Murcia, Murcia, Spain, 2015. [Google Scholar]
- Pereira, P.; Godinho, C.; Gomes, M.; Rabaça, J.E. The importance of the surroundings: Are bird communities of riparian galleries influenced by agroforestry matrices in SW Iberian Peninsula? Ann. For. Sci. 2014, 71, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.; Rabaça, J.E.; Godinho, C.; Ramos, J.A. Seasonal variation in bird species richness and abundance in riparian galleries in Southern Portugal. Acta Ornithol. 2017, 52, 69–80. [Google Scholar] [CrossRef]
- Saab, V. Importance of spatial scale to habitat use by breeding birds in riparian forests: A hierarchical analysis. Ecol. Appl. 1999, 9, 135–151. [Google Scholar] [CrossRef]
- Guareschi, S.; Abellán, P.; Laini, A.; Green, A.J.; Sánchez-Zapata, J.A.; Velasco, J.; Millán, A. Cross-taxon congruence in wetlands: Assessing the value of waterbirds as surrogates of macroinvertebrate biodiversity in Mediterranean Ramsar sites. Ecol. Indic. 2015, 49, 204–215. [Google Scholar] [CrossRef]
- Pennington, D.N.; Hansel, J.; Blair, R.B. The conservation value of urban riparian areas for landbirds during spring migration: Land cover, scale, and vegetation effects. Biol. Conserv. 2008, 141, 1235–1248. [Google Scholar] [CrossRef]
- Paracuellos, M. Estructura y conservación de las comunidades de aves en humedades del sudeste ibérico (Almería, Spain). Ph.D. Thesis, Universidad de Almería, Almería, Spain, 2001. [Google Scholar]
- Robledano, F.; Esteve-Selma, M.A.; Hernández-García, I.; Zapata, V.; Farinós-Celdrán, P.; Vives-López, R.; Martínez, J.M. Seguimiento Y Análisis de Las Actuaciones Para El Control Experimental de La Caña Común (Arundo donax) en La Región de Murcia; Comisaría de Aguas, Confederación Hidrográfica del Segura: Murcia, Spain, 2012. [Google Scholar]
- Rich, T.D. Using breeding land birds in the assessment of western riparian systems. Wildl. Soc. Bull. 2002, 30, 1128–1139. [Google Scholar]
- Larsen, S.; Sorace, A.; Mancini, L. Riparian bird communities as indicators of human impacts along Mediterranean streams. Environ. Manag. 2010, 45, 261–273. [Google Scholar] [CrossRef]
- Cavallero, L.; Raffaele, E.; Aizen, M.A. Birds as mediators of passive restoration during early post-fire recovery. Biol. Conserv. 2013, 158, 342–350. [Google Scholar] [CrossRef]
- Groom, J.D.; Grubb, T.C., Jr. Bird species associated with riparian woodland in fragmented, temperate-deciduous forest. Conserv. Biol. 2002, 16, 832–836. [Google Scholar] [CrossRef]
- Ehnström, B.; Waldén, H.W. The Protection and Management of Endangered and Declining Invertebrate Species in Swedish Woodlands; National Board of Forestry: Jönköping, Sweden, 1997. [Google Scholar]
- Fisher, A.M.; Goldney, D.C. Use by birds of riparian vegetation in an extensively fragmented landscape. Pac. Conserv. Biol. 1997, 3, 275–288. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Ribeiro, J.C.T.; Nunes-Freitas, A.F.; Fidalgo, E.C.C.; Uzêda, M.C. Forest fragmentation and impacts of intensive agriculture: Responses from different tree functional groups. PLoS ONE 2019, 14, e0212725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grantham, T.E.; Figueroa, R.; Prat, N. Water management in Mediterranean river basins: A comparison of management frameworks, physical impacts, andecological responses. Hydrobiologia 2013, 719, 451–482. [Google Scholar] [CrossRef]
- Commander, C.J. The Influence of Dams on the Abundance of Arundo donax (Giant Reed) in Riparian Corridors. Master’s Thesis, Biological Sciences-California State University, Sacramento, CA, USA, 2013. [Google Scholar]
- Catford, J.A.; Downes, B.J.; Gippel, C.J.; Vesk, P.A. Flow regulation reduces native plant cover and facilitates exotic invasion in riparian wetlands. J. Appl. Ecol. 2011, 48, 432–442. [Google Scholar] [CrossRef]
- Chen, C.; Wu, S.; Meurk, C.D.; Ma, M.; Zhao, J.; Tong, X. Effects of local and landscape factors on exotic vegetation in the riparian zone of a regulated river: Implications for reservoir conservation. Landsc. Urban Plan. 2017, 157, 45–55. [Google Scholar] [CrossRef]
- Angosto, I. Evaluación del método de cubrimiento para el control de caña (Arundo donax) en el marco de actuaciones de restauración de riberas. Bachelor’s Thesis, Environmental Sciences-Universidad de Murcia, Murcia, Spain, 2018. [Google Scholar]







| Trees | Shrubs | Herbs |
|---|---|---|
| Arbutus unedo | Crataegus monogyna | Cladium mariscus |
| Celtis australis | Ficus carica | Lonicera biflora |
| Fraxinus angustifolia | Genista spartioides | Saccharum ravennae |
| Populus alba | Juniperus oxycedrus | Scirpus holoschoenus |
| Populus nigra | Nerium oleander | Scirpus maritimus |
| Salix alba | Pistacia lentiscus | |
| Salix fragilis | Rhamnus alaternus | |
| Tamarix boveana | Rosa canina | |
| Tamarix canariensis | Salix atrocinerea | |
| Tamarix gallica | Salix purpurea lambertiana | |
| Ulmus minor | Salix triandra | |
| Sambucus nigra | ||
| Smilax aspera |
| Response Variable | Model | Year | Treatment | Year: Treatment | ||||
|---|---|---|---|---|---|---|---|---|
| Riparian Vegetation | p-Value | R2m | P | Tr. | p | Trend | p | Trend |
| Species richness | 5.5 × 10−12 | 0.33 | 1.66 × 10−8 | + | 0.45 | = | 0.33 | = |
| Riparian Quality | 0.049 | 0.08 | 0.031 | + | 0.34 | = | 0.63 | = |
| Native cover | 0.68 | - | 0.97 | = | 0.3 | = | 0.39 | = |
| A. donax stem density | 0.006 | 0.12 | 0.017 | − | 0.11 | = | 0.12 | = |
| A. donax height | 2.2 × 10−16 | 0.73 | 2 × 10−16 | − | 0.9 | = | 0.07 | = |
| A. donax cover | 0.006 | 0.08 | 0.005 | − | 0.67 | = | 0.14 | = |
| Aquatic macroinvertebrates | ||||||||
| IBMWP score | 0.003 | 0.26 | 0.004 | + | 0.47 | = | 0.67 | = |
| Family richness | 0.047 | 0.2 | 0.013 | + | 0.8 | = | 0.94 | = |
| Coleoptera richness | 0.92 | - | 0.9 | = | 0.32 | = | 0.83 | = |
| Hemiptera richness | 4.31 × 10−5 | 0.4 | 9.12 × 10−5 | + | 0.65 | = | 0.05 | = |
| Birds | ||||||||
| Species richness | 0.048 | 0.21 | 0.34 | +/= | 0.1 | = | 0.04 | Ext (+) 1, Int (=) 2 |
| Density | 0.03 | 0.11 | 0.44 | +/− | 0.58 | = | 0.02 | Ext (+), Int (−) |
| Abundance | 0.04 | 0.2 | 0.49 | +/− | 0.13 | = | 0.03 | Ext (+), Int (−) |
| Year | Treatment | Year: Treatment | ||||
|---|---|---|---|---|---|---|
| Taxa | p-Value | Temp. Trend | p | Spatial Trend (Greater Value) | p | Spatio-Temporal Trend |
| Riparian Vegetation | 0.004 | 0.004 | 0.001 | |||
| Agrostis stolonifera | Ns | = | 0.001 | Ref | 0.006 | Ref (+), Rest (=) |
| Apium nodiflorum | Ns | = | 0.001 | Ref | 0.012 | Ref (−), Rest (=) |
| Arundo donax | Ns | = | 0.002 | Rest | 0.002 | Ref (=), Rest (−), |
| Brachypodium retusum | Ns | = | 0.002 | Ref | 0.006 | Ref (+), Rest (=) |
| Carex pendula | Ns | = | 0.005 | Ref | ns | = |
| Celtis australis | 0.01 | + | ns | = | 0.042 | Ref (=), Rest (+) |
| Cistus clusii | Ns | = | 0.002 | Ref | ns | = |
| Clematis vitalba | Ns | = | 0.01 | Ref | ns | = |
| Crataegus monogyna | 0.002 | + | 0.024 | Rest | 0.002 | Ref (=), Rest (+) |
| Cyperus fuscus | Ns | = | 0.005 | Ref | ns | = |
| Daphne gnidium | Ns | = | 0.007 | Ref | 0.013 | Ref (=), Rest (+) |
| Digitalis obscura | Ns | = | 0.007 | Ref | 0.007 | Ref (+), Rest (=) |
| Dorycnium penthaphyllum | Ns | = | 0.001 | Ref | 0.004 | Ref (−), Rest (=) |
| Fraxinus angustifolia | 0.001 | + | ns | = | 0.004 | Ref (=), Rest (+) |
| Genista spartioides | 0.007 | + | ns | = | 0.041 | Ref (=), Rest (+) |
| Juniperus oxycedrus | Ns | = | 0.003 | Ref | 0.039 | Ref (−), Rest (+) |
| Mentha suaveolens | Ns | = | 0.001 | Ref | 0.008 | Ref (−), Rest (=) |
| Nerium oleander | 0.001 | + | ns | = | 0.001 | Ref (=), Rest (+) |
| Pistacia lentiscus | Ns | = | 0.003 | Ref | 0.035 | Ref (=), Rest (+) |
| Populus nigra | Ns | = | 0.004 | Ref | 0.009 | Ref (=), Rest (+) |
| Rhamnus alaternus | Ns | = | 0.004 | Ref | 0.009 | Ref (=), Rest (+) |
| Rosa canina | 0.001 | + | ns | = | 0.001 | Ref (=), Rest (+) |
| Rubia peregrina | Ns | = | 0.003 | Ref | 0.005 | Ref (=), Rest (+) |
| Rubus caesius | Ns | = | 0.002 | Ref | 0.007 | Ref (+), Rest (=) |
| Sambucus nigra | 0.001 | + | ns | = | 0.001 | Ref (=), Rest (+) |
| Samolus valerandi | Ns | = | 0.001 | Ref | ns | = |
| Salix neotricha | 0.002 | + | ns | = | 0.001 | Ref (=), Rest (+) |
| Salix purpurea | 0.001 | + | ns | = | 0.001 | Ref (=), Rest (+) |
| Smilax aspera | Ns | = | 0.001 | Ref | 0.005 | Ref (+), Rest (=) |
| Year | Treatment | Year: Treatment | ||||
|---|---|---|---|---|---|---|
| Taxa | p-Value | Temporal Trend | p | Spatial Trend (Greater Value) | p | Spatio-Temporal Trend |
| Aquatic Macroinvertebrates | 0.002 | 0.049 | 0.213 | |||
| Aeshnidae | 0.04 | + | ns | = | ns | = |
| Ancylidae | 0.024 | + | ns | = | ns | = |
| Athericidae | ns | = | 0.017 | Ref | ns | = |
| Cambaridae | 0.026 | + | 0.009 | Rest | ns | = |
| Corixidae | 0.042 | + | ns | = | ns | = |
| Dugesiidae | ns | = | 0.016 | Ref | ns | = |
| Elmidae | 0.027 | + | ns | = | ns | = |
| Gerridae | 0.012 | + | ns | = | ns | = |
| Leuctridae | ns | = | 0.017 | Ref | ns | = |
| Melanopsidae | 0.029 | + | ns | = | ns | = |
| Oligoneuriidae | ns | = | 0.018 | Ref | ns | = |
| Perlodidae | ns | = | 0.017 | Ref | ns | = |
| Planorbidae | 0.002 | − | ns | = | ns | = |
| Platycnemididae | 0.007 | + | ns | = | ns | = |
| Tabanidae | 0.001 | + | ns | = | ns | = |
| Birds | 0.012 | 0.002 | 0.046 | |||
| Aegithalos caudatus | ns | = | 0.006 | Ref | ns | = |
| Cetia cetti | ns | = | 0.002 | Ref | ns | = |
| Columba palumbus | ns | = | 0.011 | Ref | 0.014 | Ref (−), Rest (+) |
| Falco tinnunculus | ns | = | ns | = | 0.01 | Ref (−), Rest (+) |
| Fringilla coelebs | ns | = | 0.004 | Ref | ns | = |
| Jynx torquilla | 0.007 | + | ns | = | ns | = |
| Luscinia megarhynchos | ns | = | 0.006 | Ref | ns | = |
| Periparus ater | ns | = | 0.005 | Ref | ns | = |
| Picus viridis | ns | = | ns | = | 0.005 | Ref (−), Rest (+) |
| Sturnus unicolor | 0.006 | − | 0.011 | Rest | ns | = |
| Sylvia atricapilla | ns | = | 0.003 | Ref | ns | = |
| Sylvia melanocephala | ns | = | 0.002 | Ref | ns | = |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, D.; Zapata, V.; Guareschi, S.; Picazo, F.; Dettori, E.; Carbonell, J.A.; Millán, A.; Velasco, J.; Robledano, F. Short-Term Responses of Aquatic and Terrestrial Biodiversity to Riparian Restoration Measures Designed to Control the Invasive Arundo donax L. Water 2019, 11, 2551. https://doi.org/10.3390/w11122551
Bruno D, Zapata V, Guareschi S, Picazo F, Dettori E, Carbonell JA, Millán A, Velasco J, Robledano F. Short-Term Responses of Aquatic and Terrestrial Biodiversity to Riparian Restoration Measures Designed to Control the Invasive Arundo donax L. Water. 2019; 11(12):2551. https://doi.org/10.3390/w11122551
Chicago/Turabian StyleBruno, Daniel, Víctor Zapata, Simone Guareschi, Félix Picazo, Ettore Dettori, José Antonio Carbonell, Andrés Millán, Josefa Velasco, and Francisco Robledano. 2019. "Short-Term Responses of Aquatic and Terrestrial Biodiversity to Riparian Restoration Measures Designed to Control the Invasive Arundo donax L." Water 11, no. 12: 2551. https://doi.org/10.3390/w11122551
APA StyleBruno, D., Zapata, V., Guareschi, S., Picazo, F., Dettori, E., Carbonell, J. A., Millán, A., Velasco, J., & Robledano, F. (2019). Short-Term Responses of Aquatic and Terrestrial Biodiversity to Riparian Restoration Measures Designed to Control the Invasive Arundo donax L. Water, 11(12), 2551. https://doi.org/10.3390/w11122551