Pristine and Graphene-Quantum-Dots-Decorated Spinel Nickel Aluminate for Water Remediation from Dyes and Toxic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedures
2.2.1. Synthesis of NiAl2O4
2.2.2. Synthesis of GQDs and NiAl2O4/GQDs
2.3. Methods
2.4. Photocatalysis Experiments
2.5. Terephthalic Acid Probe Method
2.6. Reactive Species Scavenging
3. Results and Discussion
3.1. Structural and Morphological Study
3.1.1. NiAl2O4
3.1.2. GQDs and NiAl2O4/GQDs Composite
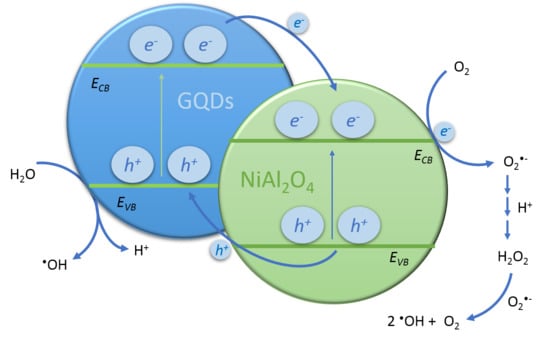
3.2. Photocatalytic Activity Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, G.; Yang, M.; Li, C.; Tan, H.; Deng, L.; Xie, S.; Xu, F.; Wang, L.; Song, Y. Preparation of spinel nickel-cobalt oxide nanowrinkles/reduced graphene oxide hybrid for nonenzymatic glucose detection at physiological level. Electrochim. Acta 2016, 220, 545–553. [Google Scholar] [CrossRef]
- Gaudon, M.; Robertson, L.C.; Lataste, E.; Duttine, M.; Ménétrier, M.; Demourgues, A. Cobalt and nickel aluminate spinels: Blue and cyan pigments. Ceram. Int. 2014, 40, 5201–5207. [Google Scholar] [CrossRef]
- Vitorino, N.M.D.; Kovalevsky, A.V.; Ferro, M.C.; Abrantes, J.C.C.; Frade, J.R. Design of NiAl2O4 cellular monoliths for catalytic applications. Mater. Des. 2017, 117, 332–337. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Yue, B.; He, H. Ni/Al2O3 catalysts derived from spinel NiAl2O4 for low-temperature hydrogenation of maleic anhydride to succinic anhydride. Chin. J. Catal. 2017, 38, 1166–1173. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Jiménez-González, C.; de Rivas, B.; Gutiérrez-Ortiz, J.I. Partial oxidation of methane to syngas on bulk NiAl2O4 catalyst. Comparison with alumina supported nickel, platinum and rhodium catalysts. Appl. Catal. Gen. 2012, 437–438, 53–62. [Google Scholar]
- Cesteros, Y.; Salagre, P.; Medina, F.; Sueiras, J. Synthesis and characterization of several Ni/NiAl2O4 catalysts active for the 1,2,4-trichlorobenzene hydrodechlorination. Appl. Catal. B Environ. 2000, 25, 213–227. [Google Scholar] [CrossRef]
- Farahani, M.D.; Dasireddy, V.D.B.C.; Friedrich, H.B. Oxidative Dehydrogenation of n-Octane over Niobium-Doped NiAl2O4: An Example of Beneficial Coking in Catalysis over Spinel. ChemCatChem 2018, 10, 2059–2069. [Google Scholar] [CrossRef]
- Maddahfar, M.; Ramezani, M.; Sadeghi, M.; Sobhani-Nasab, A. NiAl2O4 nanoparticles: Synthesis and characterization through modify sol–gel method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2015, 26, 7745–7750. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Ahmadi, F.; Eghbali-Arani, M. Different morphologies fabrication of NiAl2O4 nanostructures with the aid of new template and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2017, 28, 2415–2420. [Google Scholar] [CrossRef]
- Tangcharoen, T.; T-Thienprasert, J.; Kongmark, C. Optical properties and versatile photocatalytic degradation ability of MAl2O4 (M = Ni, Cu, Zn) aluminate spinel nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 8995–9006. [Google Scholar] [CrossRef]
- Elakkiya, V.; Agarwal, Y.; Sumathi, S. Photocatalytic activity of divalent ion (copper, zinc and magnesium) doped NiAl2O4. Solid State Sci. 2018, 82, 92–98. [Google Scholar] [CrossRef]
- Akika, F.Z.; Benamira, M.; Lahmar, H.; Tibera, A.; Chabi, R.; Avramova, I.; Suzer, Ş.; Trari, M. Structural and optical properties of Cu-substitution of NiAl2O4 and their photocatalytic activity towards Congo red under solar light irradiation. J. Photochem. Photobiol. Chem. 2018, 364, 542–550. [Google Scholar] [CrossRef]
- Jayasree, S.; Manikandan, A.; Antony, S.A.; Uduman Mohideen, A.M.; Barathiraja, C. Magneto-Optical and Catalytic Properties of Recyclable Spinel NiAl2O4 Nanostructures Using Facile Combustion Methods. J. Supercond. Nov. Magn. 2016, 29, 253–263. [Google Scholar] [CrossRef]
- Deraz, N.M. Synthesis and Characterization of Nano-Sized Nickel Aluminate Spinel Crystals. Int. J. Electrochem. Sci. 2013, 5203–5212. [Google Scholar]
- Gholami, T.; Salavati-Niasari, M.; Varshoy, S. Electrochemical hydrogen storage capacity and optical properties of NiAl2O4/NiO nanocomposite synthesized by green method. Int. J. Hydrog. Energy 2017, 42, 5235–5245. [Google Scholar] [CrossRef]
- Gholami, T.; Salavati-Niasari, M.; Salehabadi, A.; Amiri, M.; Shabani-Nooshabadi, M.; Rezaie, M. Electrochemical hydrogen storage properties of NiAl2O4/NiO nanostructures using TiO2, SiO2 and graphene by auto-combustion method using green tea extract. Renew. Energy 2018, 115, 199–207. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, H.; Jiang, Q.; Deng, Y.; Jin, H.; Kang, Q. Development of a chemical-looping combustion reactor having porous honeycomb chamber and experimental validation by using NiO/NiAl2O4. Appl. Energy 2018, 211, 259–268. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Wu, X.; Tang, D.; Cai, K.; Zhang, Q. Carbon quantum dots/Ni–Al layered double hydroxide composite for high-performance supercapacitors. RSC Adv. 2016, 6, 39317–39322. [Google Scholar] [CrossRef]
- Iguchi, S.; Teramura, K.; Hosokawa, S.; Tanaka, T. Photocatalytic conversion of CO2 in water using fluorinated layered double hydroxides as photocatalysts. Appl. Catal. Gen. 2016, 521, 160–167. [Google Scholar] [CrossRef]
- Iguchi, S.; Hasegawa, Y.; Teramura, K.; Hosokawa, S.; Tanaka, T. Preparation of transition metal-containing layered double hydroxides and application to the photocatalytic conversion of CO2 in water. J. CO2 Util. 2016, 15, 6–14. [Google Scholar] [CrossRef]
- Khodam, F.; Rezvani, Z.; Amani-Ghadim, A.R. Fabrication of a novel ZnO/MMO/CNT nanohybrid derived from multi-cationic layered double hydroxide for photocatalytic degradation of azo dye under visible light. RSC Adv. 2015, 5, 19675–19685. [Google Scholar] [CrossRef]
- Salehi, G.; Abazari, R.; Mahjoub, A.R. Visible-Light-Induced Graphitic–C3N4@Nickel–Aluminum Layered Double Hydroxide Nanocomposites with Enhanced Photocatalytic Activity for Removal of Dyes in Water. Inorg. Chem. 2018, 57, 8681–8691. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Zhang, N.; Xu, Y.-J. Synthesis of Fullerene-, Carbon Nanotube-, and Graphene-TiO2 Nanocomposite Photocatalysts for Selective Oxidation: A Comparative Study. ACS Appl. Mater. Interfaces 2013, 5, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Regulska, E.; Rivera-Nazario, D.M.; Karpinska, J.; Plonska-Brzezinska, M.E.; Echegoyen, L. Enhanced Photocatalytic Performance of Porphyrin/Phthalocyanine and Bis(4-pyridyl)pyrrolidinofullerene modified Titania. ChemistrySelect 2017, 2, 2462–2470. [Google Scholar] [CrossRef]
- Regulska, E.; Karpińska, J. Investigation of novel material for effective photodegradation of bezafibrate in aqueous samples. Environ. Sci. Pollut. Res. 2014, 21, 5242–5248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulska, E.; Karpinska, J. Investigation of Photocatalytic Activity of C60/TiO2 Nanocomposites Produced by Evaporation Drying Method. Pol. J. Environ. Stud. 2014, 23, 2175–2182. [Google Scholar]
- Hamadanian, M.; Shamshiri, M.; Jabbari, V. Novel high potential visible-light-active photocatalyst of CNT/Mo, S-codoped TiO2 hetero-nanostructure. Appl. Surf. Sci. 2014, 317, 302–311. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Fu, H.-B.; Zhu, Y.-F. Efficient TiO2 Photocatalysts from Surface Hybridization of TiO2 Particles with Graphite-like Carbon. Adv. Funct. Mater. 2008, 18, 2180–2189. [Google Scholar] [CrossRef]
- Yuan, L.; Yu, Q.; Zhang, Y.; Xu, Y.-J. Graphene–TiO2 nanocomposite photocatalysts for selective organic synthesis in water under simulated solar light irradiation. RSC Adv. 2014, 4, 15264–15270. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.-S. Solvothermal synthesis of anatase TiO2-graphene oxide nanocomposites and their photocatalytic performance. J. Alloy Compd. 2016, 688, 123–129. [Google Scholar] [CrossRef]
- Chinnusamy, S.; Kaur, R.; Bokare, A.; Erogbogbo, F. Incorporation of graphene quantum dots to enhance photocatalytic properties of anatase TiO2. Mrs Commun. 2018, 8, 137–144. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Gupta, B.K.; Kedawat, G.; Agrawal, Y.; Kumar, P.; Dwivedi, J.; Dhawan, S.K. A Novel Strategy to Enhance Ultraviolet Light Driven Photocatalysis from Graphene Quantum Dots Infilled TiO2 Nanotube Arrays. RSC Adv. 2015, 5, 10623–10631. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, S.; Tan, T.T.Y.; Xiao, F.-X. Graphene Quantum Dots (GQDs) and Its Derivatives for Multifarious Photocatalysis and Photoelectrocatalysis. Catal. Today 2018, 315, 171–183. [Google Scholar] [CrossRef]
- Page, S.E.; Arnold, W.A.; McNeill, K. Terephthalate as a Probe for Photochemically Generated Hydroxyl Radical. J. Environ. Monit. 2010, 12, 1658–1665. [Google Scholar] [CrossRef]
- Liao, Y.; Zhu, S.; Chen, Z.; Lou, X.; Zhang, D. A Facile Method of Activating Graphitic Carbon Nitride for Enhanced Photocatalytic Activity. Phys. Chem. Chem. Phys. 2015, 17, 27826–27832. [Google Scholar] [CrossRef]
- Maniammal, K.; Madhu, G.; Biju, V. X-ray Diffraction Line Profile Analysis of Nanostructured Nickel Oxide: Shape Factor and Convolution of Crystallite Size and Microstrain Contributions. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 85, 214–222. [Google Scholar] [CrossRef]
- Motahari, F.; Mozdianfard, M.R.; Soofivand, F.; Salavati-Niasari, M. NiO nanostructures: Synthesis, characterization and photocatalyst application in dye wastewater treatment. RSC Adv. 2014, 4, 27654–27660. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, W.; Wang, L.; Zhang, J.Z.; Li, Y.; Liu, X.; Li, Y. Optimizing Oxygen Functional Groups in Graphene Quantum Dots for Improved Antioxidant Mechanism. Phys. Chem. Chem. Phys. 2019, 21, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, W.Y. Partial Oxidation of Methane to Syngas over Calcined Ni–Mg/Al Layered Double Hydroxides. Catal. Lett. 2002, 83, 65–70. [Google Scholar] [CrossRef]
- Serway, R.A.; Beichner, R.J.; Jewett, J.W. Physics for Scientists and Engineers, 5th ed.; Saunders Golden Sunburst Series; Saunders College Publishing: Fort Worth, TX, USA, 2000; ISBN 978-0-03-022654-0. [Google Scholar]














| Catalyst | NiAl2O4 | NiAl2O4/GQDs | |||||
|---|---|---|---|---|---|---|---|
| Ref. | [8] | [11] | [9] | [10] | [13] | This work | This work |
| Eg/eV | 2.85 | 3.0 | 3.1 | 3.45 | 3.41 | 2.9 | 2.5 |
| Sample | Rate Constants k/h−1 | |||||
|---|---|---|---|---|---|---|
| RhB | QY | EB | MB | PH | TM | |
| NiAl2O4 | 0.068 | 0.282 | 0.354 | 1.044 | 0.401 | 0.852 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regulska, E.; Breczko, J.; Basa, A. Pristine and Graphene-Quantum-Dots-Decorated Spinel Nickel Aluminate for Water Remediation from Dyes and Toxic Pollutants. Water 2019, 11, 953. https://doi.org/10.3390/w11050953
Regulska E, Breczko J, Basa A. Pristine and Graphene-Quantum-Dots-Decorated Spinel Nickel Aluminate for Water Remediation from Dyes and Toxic Pollutants. Water. 2019; 11(5):953. https://doi.org/10.3390/w11050953
Chicago/Turabian StyleRegulska, Elzbieta, Joanna Breczko, and Anna Basa. 2019. "Pristine and Graphene-Quantum-Dots-Decorated Spinel Nickel Aluminate for Water Remediation from Dyes and Toxic Pollutants" Water 11, no. 5: 953. https://doi.org/10.3390/w11050953
APA StyleRegulska, E., Breczko, J., & Basa, A. (2019). Pristine and Graphene-Quantum-Dots-Decorated Spinel Nickel Aluminate for Water Remediation from Dyes and Toxic Pollutants. Water, 11(5), 953. https://doi.org/10.3390/w11050953