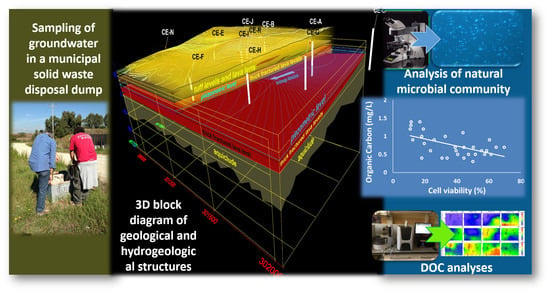
Groundwater Autochthonous Microbial Communities as Tracers of Anthropogenic Pressure Impacts: Example from a Municipal Waste Treatment Plant (Latium, Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological and Hydrogeological Setting
2.2. Digital Elevation Model
2.3. Groundwater Sampling and on Site Characterization
2.4. Chemical Analyses
2.5. Dissolved Organic Carbon (DOC)
2.6. Microbiological Analysis
2.7. Statistical Analysis
2.8. Geostatistical Analysis
3. Results
3.1. Geological and Hydrogeological Characterization
3.2. Main Chemical–Physical Parameters on Site
3.3. Chemical Analysis
3.4. Dissolved Organic Carbon
3.5. Microbial Abundance and Cell Viability
3.6. Spatial and Multi-Parameter Monitoring over 1 Year with 4 Season Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barra Caracciolo, A.; Fajardo, C.; Grenni, P.; Sacca’, M.L.; Amalfitano, S.; Ciccoli, R.; Martin, M.; Gibello, A. The role of a groundwater bacterial community in the degradation of the herbicide terbuthylazine. FEMS Microbiol. Ecol. 2010, 71, 127–136. [Google Scholar] [CrossRef] [PubMed]
- European Commission Water Statistics-Share of External Inflow from Neighbouring Territories in Renewable Freshwater Resources-Long-Term Annual Average. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Water_statistics (accessed on 9 May 2019).
- Ducci, D.; de Melo, M.T.C.; Preziosi, E.; Sellerino, M.; Parrone, D.; Ribeiro, L. Combining natural background levels (NBLs) assessment with indicator kriging analysis to improve groundwater quality data interpretation and management. Sci. Total Environ. 2016, 569–570, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, E.; Parrone, D.; Del Bon, A.; Ghergo, S. Natural background level assessment in groundwaters: Probability plot versus pre-selection method. J. Geochem. Explor. 2014, 143, 43–53. [Google Scholar] [CrossRef]
- Looser, M.; Parriaux, A.; Bensimon, M. Landfill underground pollution detection and characterization using inorganic traces. Water Res. 1999, 33, 3609–3616. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castañeda, S.S.; Sucgang, R.J.; Almoneda, R.V.; Mendoza, N.D.S.; David, C.P.C. Environmental isotopes and major ions for tracing leachate contamination from a municipal landfill in Metro Manila, Philippines. J. Environ. Radioact. 2012, 110, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Samadder, S.R.; Prabhakar, R.; Khan, D.; Kishan, D.; Chauhan, M.S. Analysis of the contaminants released from municipal solid waste landfill site: A case study. Sci. Total Environ. 2017, 580, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, E.; Frollini, E.; Zoppini, A.; Ghergo, S.; Melita, M.; Parrone, D.; Rossi, D.; Amalfitano, S. Disentangling natural and anthropogenic impacts on groundwater by hydrogeochemical, isotopic and microbiological data: Hints from a municipal solid waste landfill. Waste Manag. 2019, 84, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Gogu, R.C.; Dassargues, A. Current trends and future challenges in groundwater vulnerability assessment using overlay and index methods. Environ. Geol. 2000, 39, 549–559. [Google Scholar] [CrossRef]
- Civita, M.; De Maio, M. Assessing and mapping groundwater vulnerability to contamination: The Italian “combined” approach. Geofis. Int. 2004, 43, 513–532. [Google Scholar] [CrossRef]
- Tuxen, N.; Albrechtsen, H.-J.; Bjerg, P.L. Identification of a reactive degradation zone at a landfill leachate plume fringe using high resolution sampling and incubation techniques. J. Contam. Hydrol. 2006, 85, 179–194. [Google Scholar] [CrossRef] [PubMed]
- de Lipthay, J.R.; Tuxen, N.; Johnsen, K.; Hansen, L.H.; Albrechtsen, H.-J.; Bjerg, P.L.; Aamand, J. In Situ Exposure to Low Herbicide Concentrations Affects Microbial Population Composition and Catabolic Gene Frequency in an Aerobic Shallow Aquifer. Appl. Environ. Microbiol. 2002, 69, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R. Recent Advances in Contaminated Site Remediation. Water Air Soil Pollut. 2013, 224, 1573–2932. [Google Scholar] [CrossRef]
- Safonov, A.V.; Babich, T.L.; Sokolova, D.S.; Grouzdev, D.S.; Tourova, T.P.; Poltaraus, A.B.; Zakharova, E.V.; Merkel, A.Y.; Novikov, A.P.; Nazina, T.N. Microbial Community and in situ Bioremediation of Groundwater by Nitrate Removal in the Zone of a Radioactive Waste Surface Repository. Front. Microbiol. 2018, 9, 1985. [Google Scholar] [CrossRef] [PubMed]
- Taş, N.; Brandt, B.W.; Braster, M.; van Breukelen, B.M.; Röling, W.F.M. Subsurface landfill leachate contamination affects microbial metabolic potential and gene expression in the Banisveld aquifer. FEMS Microbiol. Ecol. 2018, 94, fiy156. [Google Scholar] [CrossRef] [PubMed]
- Guzzella, L.; Capri, E.; Di Corcia, A.; Barra Caracciolo, A.; Giuliano, G. Fate of Diuron and Linuron in a Field Lysimeter Experiment. J. Environ. Qual. 2006, 35, 312–323. [Google Scholar] [CrossRef]
- Alaoui, A.; Lipiec, J.; Gerke, H.H. A review of the changes in the soil pore system due to soil deformation: A hydrodynamic perspective. Soil Tillage Res. 2011, 115–116, 1–15. [Google Scholar] [CrossRef]
- Beeby, A. Applying Ecology; Springer: New York, NY, USA, 1994; ISBN 978-0-412-44470-8. [Google Scholar]
- Calow, P. Evolution, ecology and environmental stress. Biol. J. Linn. Soc. 1989, 37, 1. [Google Scholar] [CrossRef]
- Capelli, G.; Mazza, R. Water criticality in the Colli Albani (Rome, Italy). G. Geol. Appl. 2005, 1, 261–271. [Google Scholar]
- Hansen, J.E.; Lacis, A.A.; Lee, P.; Wang, W.-C. Climatic effects of atmospheric aerosols. Ann. N. Y. Acad. Sci. 1980, 338, 575–587. [Google Scholar] [CrossRef]
- Capelli, G.; Mazza, R.; Gazzetti, C. Strumenti e Strategie per la Tutela e uso Compatibile Della Risorsa Idrica nel Lazio. Gli Acquiferi Vulcanici; Pitagora: Bologna, Italy, 2005; ISBN 978-8837114503. [Google Scholar]
- Tatti, F.; Petrangeli Papini, M.; Torretta, V.; Mancini, G.; Boni, M.R.; Viotti, P. Experimental and numerical evaluation of Groundwater Circulation Wells as a remediation technology for persistent, low permeability contaminant source zones. J. Contam. Hydrol. 2019, 222, 89–100. [Google Scholar] [CrossRef]
- Dagan, G.; Neuman, S.P. Subsurface Flow and Transport: A Stochastic Approach-International Hydrology Series; Cambridge University Press: Cambridge, UK, 1997; ISBN 9780521020091. [Google Scholar]
- Sibson, R.H. Crustal stress, faulting and fluid flow. Geol. Soc. Lond. Spec. Publ. 1994, 78, 69–84. [Google Scholar] [CrossRef]
- Fischer, M.P.; Jackson, P.B. Stratigraphic controls on eformation patterns in fault-related folds: A detachment fold example from the Sierra Madre Oriental, northeast Mexico. J. Struct. Geol. 1999, 21, 613–633. [Google Scholar] [CrossRef]
- Chester, J.S. Mechanical stratigraphy and fault–fold interaction, Absaroka thrust sheet, Salt River Range, Wyoming. J. Struct. Geol. 2003, 25, 1171–1192. [Google Scholar] [CrossRef]
- Engelder, T.; Marshak, S. Disjunctive cleavage formed at shallow depths in sedimentary rocks. J. Struct. Geol. 1985, 7, 327–343. [Google Scholar] [CrossRef]
- Jamison, W.R. Stress controls on fold thrust style. In Thrust Tectonics; Springer: Dordrecht, The Netherlands, 1992; pp. 155–164. [Google Scholar]
- United Nations World Commission on Environment and Development-Brundtland Commission. Our Common Future, Report of the World Commission on Environment and Development, World Commission on Environment and Development; Oxford University Press: Oxford, UK, 1987; Volume 1, Doc. 149; ISBN 019282080X. [Google Scholar]
- Parliament, E. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Union 2000, 12, 1–51. [Google Scholar]
- Gilbert, J.A.; Steele, J.A.; Caporaso, J.G.; Steinbrück, L.; Reeder, J.; Temperton, B.; Huse, S.; McHardy, A.C.; Knight, R.; Joint, I.; et al. Defining seasonal marine microbial community dynamics. ISME J. 2012, 6, 298–308. [Google Scholar] [CrossRef]
- Grenni, P.; Gibello, A.; Barra Caracciolo, A.; Fajardo, C.; Nande, M.; Vargas, R.; Saccà, M.L.; Martinez-Iñigo, M.J.; Ciccoli, R.; Martín, M. A new fluorescent oligonucleotide probe for in situ detection of s-triazine-degrading Rhodococcus wratislaviensis in contaminated groundwater and soil samples. Water Res. 2009, 43, 2999–3008. [Google Scholar] [CrossRef]
- Amalfitano, S.; Del Bon, A.; Zoppini, A.; Ghergo, S.; Fazi, S.; Parrone, D.; Casella, P.; Stano, F.; Preziosi, E. Groundwater geochemistry and microbial community structure in the aquifer transition from volcanic to alluvial areas. Water Res. 2014, 65, 384–389. [Google Scholar] [CrossRef]
- Danielopol, D.L.; Griebler, C. Changing Paradigms in Groundwater Ecology-from the “Living Fossils” Tradition to the “New Groundwater Ecology”. Int. Rev. Hydrobiol. 2008, 93, 565–577. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef] [Green Version]
- Seibert, J.; Bishop, K.; Rodhe, A.; McDonnell, J.J. Groundwater dynamics along a hillslope: A test of the steady state hypothesis. Water Resour. Res. 2003, 39, 1014. [Google Scholar] [CrossRef]
- Henriksen, H.J.; Rasmussen, P.; Brandt, G.; von Bülow, D.; Jensen, F.V. Public participation modelling using Bayesian networks in management of groundwater contamination. Environ. Model. Softw. 2007, 22, 1101–1113. [Google Scholar] [CrossRef]
- Yagi, J.M.; Madsen, E.L. Diversity, Abundance, and Consistency of Microbial Oxygenase Expression and Biodegradation in a Shallow Contaminated Aquifer. Appl. Environ. Microbiol. 2009, 75, 6478–6487. [Google Scholar] [CrossRef] [Green Version]
- Haack, S.K.; Fogarty, L.R.; West, T.G.; Alm, E.W.; McGuire, J.T.; Long, D.T.; Hyndman, D.W.; Forney, L.J. Spatial and temporal changes in microbial community structure associated with recharge-influenced chemical gradients in a contaminated aquifer. Environ. Microbiol. 2004, 6, 438–448. [Google Scholar] [CrossRef]
- Griebler, C.; Lueders, T. Microbial biodiversity in groundwater ecosystems. Freshw. Biol. 2009, 54, 649–677. [Google Scholar] [CrossRef]
- Hancock, P.J.; Boulton, A.J.; Humphreys, W.F. Aquifers and hyporheic zones: Towards an ecological understanding of groundwater. Hydrogeol. J. 2005, 13, 98–111. [Google Scholar] [CrossRef]
- Humphreys, W.F. Hydrogeology and groundwater ecology: Does each inform the other? Hydrogeol. J. 2009, 17, 5–21. [Google Scholar] [CrossRef]
- Peccerillo, A. Plio-Quaternary Volcanism in Italy: Petrology, Geochemistry, Geodynamics; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; p. 370. ISBN 13 978-3-540-25885-8. [Google Scholar]
- De Rita, D.; Funiciello, R.; Parotto, M. Geological Map of the Colli Albani Volcanic Complex (Carta geologica del Complesso vulcanico dei Colli Albani); Consiglio Nazionale delle Ricerche: Rome, Italy, 1988; Available online: http://repositories.dst.unipi.it/index.php/carte/item/29-carta-geologica-del-complesso-vulcanico-dei-colli-albani-vulcano-laziale (accessed on 10 May 2019).
- Marra, F.; Karner, D.B.; Freda, C.; Gaeta, M.; Renne, P. Large mafic eruptions at Alban Hills Volcanic District (Central Italy): Chronostratigraphy, petrography and eruptive behavior. J. Volcanol. Geotherm. Res. 2009, 179, 217–232. [Google Scholar] [CrossRef]
- Gaeta, M.; Freda, C.; Marra, F.; Di Rocco, T.; Gozzi, F.; Arienzo, I.; Giaccio, B.; Scarlato, P. Petrology of the most recent ultrapotassic magmas from the Roman Province (Central Italy). Lithos 2011, 127, 298–308. [Google Scholar] [CrossRef]
- Capelli, G.; Mastrorillo, L.; Mazza, R.; Petitta, M. Map of the Hydrogeological Units of the Lazio Region (Carta delle Unità Idrogeologiche della Regione Lazio, scala 1:250.000; Regione Lazio, S.EL.C.A.: Firenze, Italy, 2012; Available online: http://www.regione.lazio.it/binary/rl_main/tbl_documenti/AMB_PBL_SIRDIS_Carta_Unita_in_scala_1a250.000_LD.pdf (accessed on 10 May 2019).
- Capelli, G.; Mastrorillo, L.; Mazza, R.; Petitta, M.; Baldoni, T.; Banzato, F.; Cascone, D.; Di Salvo, C.; La Vigna, F.; Taviani, S.; et al. Hydrogeological Map of the Lazio Region (Carta Idrogeologica del Territorio della Regione Lazio), (4 sheets); Regione Lazio: Rome, Italy, 2016. [Google Scholar]
- Cinti, D.; Procesi, M.; Tassi, F.; Montegrossi, G.; Sciarra, A.; Vaselli, O.; Quattrocchi, F. Fluid geochemistry and geothermometry in the western sector of the Sabatini Volcanic District and the Tolfa Mountains (Central Italy). Chem. Geol. 2011, 284, 160–181. [Google Scholar] [CrossRef]
- Cinti, D.; Poncia, P.P.; Brusca, L.; Tassi, F.; Quattrocchi, F.; Vaselli, O. Spatial distribution of arsenic, uranium and vanadium in the volcanic-sedimentary aquifers of the Vicano–Cimino Volcanic District (Central Italy). J. Geochem. Explor. 2015, 152, 123–133. [Google Scholar] [CrossRef]
- Preziosi, E.; Rossi, D.; Parrone, D.; Ghergo, S. Groundwater chemical status assessment considering geochemical background: An example from Northern Latium (Central Italy). Rend. Lincei 2016, 27, 59–66. [Google Scholar] [CrossRef]
- Moore, I.D.; Grayson, R.B.; Ladson, A.R. Digital terrain modelling: A review of hydrological, geomorphological, and biological applications. Hydrol. Process. 1991, 5, 3–30. [Google Scholar] [CrossRef]
- Moore, I.D.; Gessler, P.E.; Nielsen, G.A.; Peterson, G.A. Soil Attribute Prediction Using Terrain Analysis. Soil Sci. Soc. Am. J. 1993, 57, 443–452. [Google Scholar] [CrossRef]
- Wise, S.M. Effect of differing DEM creation methods on the results from a hydrological model. Comput. Geosci. 2007, 33, 1351–1365. [Google Scholar] [CrossRef]
- Vaze, J.; Teng, J.; Spencer, G. Impact of DEM accuracy and resolution on topographic indices. Environ. Model. Softw. 2010, 25, 1086–1098. [Google Scholar] [CrossRef]
- Italian Ministry of the Environment. Legislative Decree no. 152, 2006, Rules in environmental field. Ital. Off. J. 2006, 88, 425. Available online: https://www.gazzettaufficiale.it/eli/gu/2006/04/14/88/so/96/sg/pdf (accessed on 15 May 2019).
- Borkowski, A.S.; Kwiatkowska-Malina, J. Geostatistical modelling as an assessment tool of soil pollution based on deposition from atmospheric air. Geosci. J. 2017, 21, 645–653. [Google Scholar] [CrossRef]
- Chang, T.; Shyu, G.; Lin, Y.; Chang, N. Geostattstical analysis of soil arsenic content in taiwan. J. Environ. Sci. Heal. Part A 1999, 34, 1485–1501. [Google Scholar] [CrossRef]
- Brenning, A. Geostatistics without stationarity assumptions within Geographical Information Systems. Freib. Online Geosci. 2001, 102. Available online: http://www.geo.tu-freiberg.de/fog/FOG_Vol_6.pdf (accessed on 10 March 2019). [CrossRef]
- Frollini, E.; Rossi, D.; Rainaldi, M.; Parrone, D.; Ghergo, S.; Preziosi, E. A proposal for groundwater sampling guidelines: Application to a case study in southern Latium. Rend. Online Della Soc. Geol. Ital. 2019, 47, 46–51. [Google Scholar] [CrossRef]
- APAT-IRSA CNR (Agenzia per la Protezione Dell’ambiente e per i Servizi Tecnici-Consiglio Nazionale delle Ricerche-Istituto di Ricerca sulle Acque). Metodi Analitici per le Acque; Belli, M., Centioli, D., Zorzi, P., Umberto, S., Capri, S., Pagnotta, R., Pettine, M., Eds.; APAT: Rome, Italy, 2003; ISBN 88-448-0083-7. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Waste Waters, 23rd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Eds.; American Public Health Associatio; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Patrolecco, L.; Ademollo, N.; Grenni, P.; Tolomei, A.; Barra Caracciolo, A.; Capri, S. Simultaneous determination of human pharmaceuticals in water samples by solid phase extraction and HPLC with UV-fluorescence detection. Microchem. J. 2013, 107, 165–171. [Google Scholar] [CrossRef]
- Ademollo, N.; Patrolecco, L.; Polesello, S.; Valsecchi, S.; Wollgast, J.; Mariani, G.; Hanke, G. The analytical problem of measuring total concentrations of organic pollutants in whole water. TrAC Trends Anal. Chem. 2012, 36, 71–81. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Giuliano, G.; Grenni, P.; Cremisini, C.; Ciccoli, R.; Ubaldi, C. Effect of urea on degradation of terbuthylazine in soil. Environ. Toxicol. Chem. 2005, 24, 1035–1040. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Giuliano, G.; Grenni, P.; Guzzella, L.; Pozzoni, F.; Bottoni, P.; Fava, L.; Crobe, A.; Orrù, M.; Funari, E. Degradation and leaching of the herbicides metolachlor and diuron: A case study in an area of Northern Italy. Environ. Pollut. 2005, 134, 525–534. [Google Scholar] [CrossRef]
- Isaaks, E.H.; Srivastava, R.M. An Introduction to Applied Geostatistics; Oxford University Press: New York, NY, USA, 1989; Volume 17, ISBN 0-19-505012-6. [Google Scholar]
- Cressie, N. The origins of kriging. Math. Geol. 1990, 22, 239–252. [Google Scholar] [CrossRef]
- Cressie, N.A.C. Statistics for Spatial Data; Wiley Series in Probability and Statistics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1993; ISBN 9781119115151. [Google Scholar]
- Burrough, P.A.; McDonnell, R.A. Principles of Geographical Information Systems; Oxford University Press: Oxford, UK, 1999; ISBN 019-8233655. [Google Scholar]
- Wu, Z.; Guo, F.; Li, J. The 3D modelling techniques of digital geological mapping. Arab. J. Geosci. 2019, 12, 467. [Google Scholar] [CrossRef]
- Kaufmann, O.; Martin, T. 3D geological modelling from boreholes, cross-sections and geological maps, application over former natural gas storages in coal mines. Comput. Geosci. 2008, 34, 279–290. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Zhu, H.; Liu, J. Geomodeling with integration of multi-source data by Bayesian kriging in underground space. Tongji Daxue Xuebao/Journal Tongji Univ. 2014, 42, 406–412. [Google Scholar]
- Wu, Z.; Guo, F.; Liu, Z.; Hou, M.; Luo, J. Technology and method of multi-data merging in 3D geological modelling. J. Jilin Univ. Earth Sci. Ed. 2016, 6, 1895–1913. [Google Scholar]
- Berg, R.C.; Greenpool, M.R. Stack-Unit Geologic Mapping: Color-Coded and Computer-Based Methodology; Department of Energy and Natural Resources, Illinois State Geological Survey Library: Champaign, IL, USA, 1993; pp. 1–11. [Google Scholar]
- Berg, R.C.; Kempton, J.P. Stack-unit Mapping of Geologic Materials in Illinois to a Depth of 15 m; Department of Energy and Natural Resources, Illinois State Geological Survey Library: Champaign, IL, USA, 1988. [Google Scholar]
- Angelone, M.; Cremisini, C.; Piscopo, V.; Proposito, M.; Spaziani, F. Influence of hydrostratigraphy and structural setting on the arsenic occurrence in groundwater of the Cimino-Vico volcanic area (central Italy). Hydrogeol. J. 2009, 17, 901–914. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Grenni, P.; Falconi, F.; Caputo, M.C.; Ancona, V.; Uricchio, V.F. Pharmaceutical waste disposal: Assessment of its effects on bacterial communities in soil and groundwater. Chem. Ecol. 2011, 27, 43–51. [Google Scholar] [CrossRef]
- Saccà, M.L.; Ferrero, V.E.V.; Loos, R.; Di Lenola, M.; Tavazzi, S.; Grenni, P.; Ademollo, N.; Patrolecco, L.; Huggett, J.; Barra Caracciolo, A.; et al. Chemical mixtures and fluorescence in situ hybridization analysis of natural microbial community in the Tiber river. Sci. Total Environ. 2019, 673, 7–19. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Patrolecco, L.; Grenni, P.; Di Lenola, M.; Ademollo, N.; Rauseo, J.; Rolando, L.; Spataro, F.; Plutzer, J.; Monostory, K.; et al. Chemical mixtures and autochthonous microbial community in an urbanized stretch of the River Danube. Microchem. J. 2019, 147, 985–994. [Google Scholar] [CrossRef]
- Bi, E.; Liu, Y.; He, J.; Wang, Z.; Liu, F. Screening of Emerging Volatile Organic Contaminants in Shallow Groundwater in East China. Ground Water Monit. Remediat. 2012, 32, 53–58. [Google Scholar] [CrossRef]






| Piezometer | Maximum Depth from Surface (m) | Piezometer-Top/Bottom (m) (a.s.l.) | Diameter (mm) | Static Level (m) (a.s.l.) | Range | |||
|---|---|---|---|---|---|---|---|---|
| I September | II February | III July | IV October | |||||
| CE-A | 153 | 156.39/3.39 | 150 | 63.5 | 63.8 | 63.8 | 64.2 | 0.7 |
| CE-B | 100 | 140.74/40.74 | 150 | 59.6 | 59.7 | 60 | 60 | 0.4 |
| CE-C | 142 | 142.58/0.58 | 250 | 57.1 | 57.5 | 57.8 | 57.6 | 0.7 |
| CE-D | 158 | 166.31/8.31 | 180 | 68.8 | 68.9 | 69.4 | 69.3 | 0.6 |
| CE-E | 15 | 134.52/119.52 | 75 | - | - | - | - | - |
| CE-F | 90 | 134.04/44.04 | 75 | 54.2 | 54.8 | 56.1 | 56.1 | 1.9 |
| CE-G | 120 | 155.82/35.82 | 75 | 55.5 | 53.8 | 62.8 | 57.2 | 7.3 |
| CE-H | 115 | 137.31/20.31 | 75 | 54.9 | 54.2 | 58.9 | 56.4 | 4.7 |
| CE-I | 125 | 143.96/16.96 | 170 | 56.2 | 56.6 | 56.8 | 56.6 | 0.4 |
| Group of Organic Contaminants | Chemicals |
|---|---|
| Polycyclic Aromatic Hydrocarbons (PAHs) | Naphthalene, Acenaphthene, Fluorene, Phenanthrene, Anthracene, Fluoranthene, Pyrene, Benzo(a)Anthracene, Chrysene, Benzo(b)fluoranthene, Benzo(k)fluoranthene, Benzo(a)pyrene, Dibenz(a,h)anthracene, Benzo(g,h,i)perylene, Indeno(1,2,3-c,d)pyrene, Dibenzo(a,e) pyrene, Dibenzo(a,i) pyrene, Dibenzo(a,h) pyrene, Dibenzo(a,l) pyrene |
| Volatile Organic Compounds (VOCs) | Trichloromethane, Tribromomethane, 1,2-Dibromoethane, Dibromochloromethane, Bromodichloromethane, 1,2-Dichloroethane, 1,1-Dichloroethane, 1,2-Dichloropropane, 1,1,2-Trichloroethane, 1,2,3-Trichloropropane, 1,1,2,2-Tetrachloroethane, Chloroethene, Chloromethane, 1,2-Dichloroethene, 1,1-Dichloroethene, Trichloroethylene, Tetrachloroethylene, Hexachloro-1,3-butadiene, Chlorobenzene, 1,2-Dichlorobenzene, 1,3-Dichlorobenzene, 1,4-Dichlorobenzene, 1,2,4-Trichlorobenzene, 1,2,3-Trichlorobenzene, n-Hexane, Benzene, Ethylbenzene, Styrene, Toluene, ortho-Xylene, meta-Xylene, para-Xylene, (1-methylethyl)benzene, Propylbenzene, Methylbenzene, Bromobenzene, Phenylamine, Diphenylamine, o-Toluidine, prop-2-enamide, pyridine-3-carboxylic acid, 1,2-Dinitrobenzene, 1,3-Dinitrobenzene, 1-methyl-2,4-dinitro benzene, n-Nitrotoluene, 1-Chloro-2-nitrobenzene, 1-Chloro-3-nitrobenzene,1-Chloro-4-nitrobenzene, 1,2,3-Trichloropropane, Dichloromethane, Bromomethane, Tetrachloromethane, Chloroethane, Dichlorodifluoromethane, Trichlorofluoromethane, Bromoethene, 1-bromo-2-chloroethane, Dichlorobutane, 1,1,1-Trichloroethane, 1,1,1,2-Tetrachloroethane, Bromodichloroethane, 4-Bromochlorobenzene, 1,1-Dichlorocycloutane, 1,4-Dichlorobut-2-ene, 2-Methoxy-2-methylpropane, Diiodomethane, n- Propylbenzene |
| Chlorinated Pesticides | Dichlorodiphenyldichloroethylene (DDE) Gamma-hexachlorocyclohexane (γ HCH) |
| Piezometer | Conductivity (µS/cm) | Dissolved Oxygen (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| I September | II February | III July | IV October | I September | II February | III July | IV October | |
| CE-A | 704 | 1404 | 789 | 758 | 9.14 | 8.30 | 8.8 | 4.3 |
| CE-B | 1029 | 1022 | 1028 | 1039 | 3.3 | 3.50 | 3.2 | 3.3 |
| CE-C | 855 | 905 | 847 | 840 | 8.1 | 5.2 | 4.0 | 7.5 |
| CE-D | 718 | 1412 | 713 | 710 | 8.87 | 8.9 | 8.9 | 8.8 |
| CE-E | 713 | 707 | 709 | 718 | 6.8 | 6.58 | 5.1 | 6.4 |
| CE-F | 1039 | 1040 | 1035 | 1049 | 4.4 | 3.64 | 3.7 | 3.7 |
| CE-G | - | 850 | 862 | - | - | 6.97 | 5.1 | - |
| CE-H | 850 | 843 | 790 | 798 | 3.54 | 6.28 | 7.5 | 7.4 |
| CE-I | 769 | 792 | 770 | 774 | 6.5 | 6.73 | 6.7 | 7.3 |
| Ions | Min | Max | Legal limits * | >Limits | Piezometers |
|---|---|---|---|---|---|
| Fluorides | 0.86 | 6.64 | 1.5 mg/L | 11 | CE-C; CE-E; CE-H; CE-I |
| Nitrites | 0 | 0 | 0.5 mg/L | 0 | |
| Sulphates | 19.1 | 40.39 | 250 mg/L | 0 | |
| Be | 0.1 | 0.9 | 4 μg/L | 0 | |
| B | 114 | 803.54 | 1000 μg/L | 0 | |
| Al | 3.1 | 1718 | 200 μg/L | 8 | CE-E; CE-F; CE-G; CE-H |
| Cr Tot | 0.8 | 3.7 | 50 μg/L | 0 | |
| Cr (VI) | <0.5 | <0.5 | 5 μg/L | 0 | |
| Mn | 0.2 | 46.2 | 50 μg/L | 0 | |
| Fe | 7.5 | 543 | 200 μg/L | 3 | CE-G; CE-H |
| As | 8.3 | 26.58 | 10 μg/L | 28 | CE-A; CE-B; CE-C; CE-E; CE-F; CE-G; CE-H; CE-I |
| Ni | 0.3 | 7.8 | 20 μg/L | 0 | |
| Cu | 0.21 | 4.53 | 1000 μg/L | 0 | |
| Co | 0.3 | 2.5 | 50 μg/L | 0 | |
| Zn | 2.5 | 838 | 3000 μg/L | 0 | |
| Se | 0.1 | 0.6 | 10 μg/L | 0 | |
| Ag | <0.1 | <0.1 | 10 μg/L | 0 | |
| V | 30.2 | 49.8 | 50 μg/L | 0 | |
| Cd | 0.11 | 0.15 | 5 μg/L | 0 | |
| Sb | 0.2 | 0.4 | 5 μg/L | 0 | |
| Hg | <0.1 | 1 μg/L | 0 | ||
| Pb | 0.1 | 1.5 | 10 μg/L | 0 |
| Piezometer | I September (µg/L) | II February (µg/L) | III July (µg/L) | IV October (µg/L) |
|---|---|---|---|---|
| CE-A | 0.398 | 0.122 | 0.434 | 0.287 |
| CE-B | 0.813 | 0.238 | 1.08 | 0.597 |
| CE-C | 0.508 | 0.129 | 0.92 | 0.353 |
| CE-D | 0.312 | 0.049 | 0.674 | 0.259 |
| CE-E | 0.291 | 0.061 | 0.465 | 0.236 |
| CE-F | 0.667 | 0.529 | 1.579 | - |
| CE-G | 0.646 | 0.163 | 0.347 | 0.179 |
| CE-H | 0.651 | 0.121 | - | 0.546 |
| CE-I | 0.398 | 0.219 | 1.12 | 0.288 |
| Piezometer | DOC (mg/L) | |||||
|---|---|---|---|---|---|---|
| I September | II February | III July | IV October | Average | ±se | |
| CE-A | 0.4 | 0.9 | 0.5 | 0.5 | 0.6 | ±0.1 |
| CE-B | 1.4 | 1.3 | 0.9 | 0.7 | 1.1 | ±0.2 |
| CE-C | 0.5 | 1.2 | 0.6 | 0.4 | 0.7 | ±0.2 |
| CE-D | 0.5 | 0.7 | 0.6 | 0.4 | 0.6 | ±0.1 |
| CE-E | 0.3 | 0.7 | 0.7 | 0.6 | 0.6 | ±0.1 |
| CE-F | 1.0 | 1.2 | 1.2 | 1.0 | 1.1 | ±0.0 |
| CE-G | 0.4 | 0.8 | 1.4 | 0.6 | 0.8 | ±0.3 |
| CE-H | 0.5 | 1.1 | 2.0 | 0.7 | 1.1 | ±0.3 |
| CE-I | 0.4 | 0.9 | 0.7 | 0.6 | 0.7 | ±0.1 |
| Piezometer | Microbial Abundance (No. Cells/mL) | |||||
|---|---|---|---|---|---|---|
| I September | II February | III July | IV October | Average | ±se | |
| CE-A | 6.9 × 103 | 8.8 × 104 | 5.2 × 104 | 1.4 × 105 | 7.2 × 104 | ±2.9 × 104 |
| CE-B | 1.7 × 104 | 3.1 × 104 | 2.9 × 104 | 6.6 × 104 | 3.6 × 104 | ±1.1 × 104 |
| CE-C | 9.2 × 103 | 3.1 × 104 | 9.0 × 103 | 2.6 × 104 | 1.9 × 104 | ±5.7 × 103 |
| CE-D | 1.0 × 104 | 1.4 × 104 | 1.2 × 104 | 2.2 × 104 | 1.4 × 104 | ±2.5 × 103 |
| CE-E | 1.5 × 104 | 2.6 × 104 | 1.9 × 104 | 1.1 × 105 | 4.2 × 104 | ±2.2 × 104 |
| CE-F | 5.3 × 103 | 2.6 × 104 | 6.6 × 104 | 2.5 × 105 | 8.8 × 104 | ±5.7 × 104 |
| CE-G | - | 1.7 × 104 | 1.7 × 105 | - | 9.4 × 104 | ±7.7 × 104 |
| CE-H | 2.5 × 104 | 6.5 × 104 | 1.5 × 104 | 1.2 × 106 | 3.3 × 105 | ±2.9 × 105 |
| CE-I | 1.2 × 104 | 5.8 × 104 | 1.5 × 104 | 3.5 × 104 | 3.0 × 104 | ±1.1 × 104 |
| Piezometer | Cell Viability (%) | |||||
|---|---|---|---|---|---|---|
| I September | II February | III July | IV October | Average | ±se | |
| CE-A | 33.8 | 12.3 | 51.5 | 47.2 | 36.2 | ±8.8 |
| CE-B | 11.3 | 11.2 | 60.3 | 50.2 | 33.2 | ±12.9 |
| CE-C | 61.4 | 10.7 | 58.6 | 30.6 | 40.3 | ±12.1 |
| CE-D | 57.3 | 44.3 | 23.5 | 44.3 | 42.3 | ±7.0 |
| CE-E | 50.0 | 38.9 | 64.5 | 39.5 | 48.2 | ±6.0 |
| CE-F | 18.0 | 19.0 | 37.1 | 32.7 | 26.7 | ±4.8 |
| CE-G | - | 19.6 | 16.8 | - | 18.2 | ±1.4 |
| CE-H | 40.4 | 43.8 | - | 18 | 34.1 | ±8.1 |
| CE-I | 34.9 | 25.7 | 69.5 | 55.5 | 46.4 | ±12.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, D.; Barra Caracciolo, A.; Grenni, P.; Cattena, F.; Di Lenola, M.; Patrolecco, L.; Ademollo, N.; Ciannarella, R.; Mascolo, G.; Ghergo, S. Groundwater Autochthonous Microbial Communities as Tracers of Anthropogenic Pressure Impacts: Example from a Municipal Waste Treatment Plant (Latium, Italy). Water 2019, 11, 1933. https://doi.org/10.3390/w11091933
Rossi D, Barra Caracciolo A, Grenni P, Cattena F, Di Lenola M, Patrolecco L, Ademollo N, Ciannarella R, Mascolo G, Ghergo S. Groundwater Autochthonous Microbial Communities as Tracers of Anthropogenic Pressure Impacts: Example from a Municipal Waste Treatment Plant (Latium, Italy). Water. 2019; 11(9):1933. https://doi.org/10.3390/w11091933
Chicago/Turabian StyleRossi, David, Anna Barra Caracciolo, Paola Grenni, Flavia Cattena, Martina Di Lenola, Luisa Patrolecco, Nicoletta Ademollo, Ruggiero Ciannarella, Giuseppe Mascolo, and Stefano Ghergo. 2019. "Groundwater Autochthonous Microbial Communities as Tracers of Anthropogenic Pressure Impacts: Example from a Municipal Waste Treatment Plant (Latium, Italy)" Water 11, no. 9: 1933. https://doi.org/10.3390/w11091933
APA StyleRossi, D., Barra Caracciolo, A., Grenni, P., Cattena, F., Di Lenola, M., Patrolecco, L., Ademollo, N., Ciannarella, R., Mascolo, G., & Ghergo, S. (2019). Groundwater Autochthonous Microbial Communities as Tracers of Anthropogenic Pressure Impacts: Example from a Municipal Waste Treatment Plant (Latium, Italy). Water, 11(9), 1933. https://doi.org/10.3390/w11091933