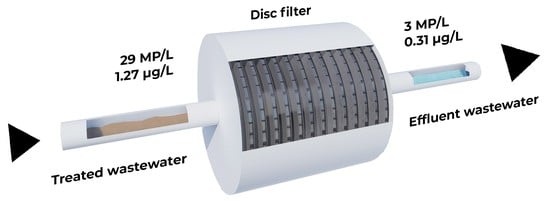
Removal of >10 µm Microplastic Particles from Treated Wastewater by a Disc Filter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sample Preparation
2.3. Analysis
2.4. Statistics
3. Results
3.1. Removal Efficiency
3.2. Polymer Composition of MPs
3.3. Particle Size
4. Discussion
4.1. Removal Efficiency of the Disc Filter
4.2. Particle Size
4.3. Polymer Composition
4.4. The Role of Disc Filters in MP Removal
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Burton, H.; Fitch, S.; Schulz, M.; van den Hoff, J. Daily accumulation rates of marine debris on sub-Antarctic island beaches. Mar. Pollut. Bull. 2013, 66, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Cozar, A.; Echevarria, F.; Gonzalez-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernandez-Leon, S.; Palma, A.T.; Navarro, S.; Garcia-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Villarrubia-Gómez, P.; Cornell, S.E.; Fabres, J. Marine plastic pollution as a planetary boundary threat—The drifting piece in the sustainability puzzle. Mar. Policy 2017. [Google Scholar] [CrossRef]
- Burns, E.E.; Boxall, A.B.A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796. [Google Scholar] [CrossRef] [Green Version]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [Green Version]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2014; Available online: https://www.diva-portal.org/smash/get/diva2:773505/FULLTEXT01.pdf (accessed on 17 September 2019).
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142. [Google Scholar] [CrossRef]
- Miljøstyrelsen. Punktkilder 2016; The Danish Environmental Protection Agency: Odense, Denmark, 2018; Available online: https://www2.mst.dk/Udgiv/publikationer/2018/04/978-87-93614-44-4.pdf (accessed on 17 September 2019).
- Norén, K.; Magnusson, K.; Westling, K.; Olshammar, M. Report Concerning Techniques to Reduce Litter in Waste Water and Storm Water; Swedish Meteorological and Hydrological Institute: Norrköping, Sweden, 2016; Available online: https://admin-sm.transfers.se/app/uploads/2016/10/SMED-Report_-193-2016-BAT-Microlitter.pdf (accessed on 17 September 2019).
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123. [Google Scholar] [CrossRef]
- Liu, F.; Olesen, K.B.; Borregaard, A.R.; Vollertsen, J. Microplastics in urban and highway stormwater retention ponds. Sci. Total Environ. 2019, 671. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 17 September 2019).
- Dinno, A. dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums. 2017. Available online: https://CRAN.R-project.org/package=dunn.test (accessed on 19 March 2019).
- Olesen, K.B.; Stephansen, D.A.; Alst, N.; Van Vollertsen, J. Microplastics in a Stormwater Pond. Water 2019, 11, 1466. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Kuczera, M.; Mintenig, S.; Lorenz, C.; Gerdts, G. Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ. Chem. 2015, 12, 563–581. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef]
- Primpke, S.; Dias, P.A.; Gerdts, G. Automated identification and quantification of microfibres and microplastics. Anal. Methods 2019, 11, 2138–2147. [Google Scholar] [CrossRef]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef] [Green Version]
- Gómez, M.; de la Rua, A.; Garralón, G.; Plaza, F.; Hontoria, E.; Gómez, M.A. Urban wastewater disinfection by filtration technologies. Desalination 2006, 190, 16–28. [Google Scholar] [CrossRef]
- Magnusson, K.; Jörundsdóttir, H.; Norén, F.; Lloyd, H.; Talvitie, J.; Setälä, O. Microlitter in Sewage Treatment Systems; TemaNord, Nordic Council of Ministers: Copenhagen K, Denmark, 2016; Available online: https://norden.diva-portal.org/smash/get/diva2:923936/FULLTEXT01.pdf (accessed on 17 September 2019). [CrossRef]
- Nielsen, K.; Mørch-Madsen, A.; Mikkelsen, P.S.; Eriksson, E. Effect of Disc Filtration with and without Addition of Flocculent on Nano-and Micro-Particles and Their Associated Polycyclic Aromatic Hydrocarbons in Stormwater. Water 2015, 7, 1306–1323. [Google Scholar] [CrossRef]
- Fylypchuk, V.; Induchny, S.; Pearce, P.; Fylypchuk, L.; Martynov, S. Application of Expanded Polystyrene Filter for Tertiary Treatment of Domestic Waste Effluent in the UK. J. Water Land Dev. 2017, 35, 41–47. [Google Scholar] [CrossRef]




| Polymers | ||||
| Polyethylene | Vinyl copolymer | Styrene butadiene | Poly(tetrafluoroethylene) | Acrylic paints |
| Polypropylene | Ethylene vinyl acetate | Acrylonitrile butadiene styrene | Ethylene propylene diene monomer (EPDM) | PEBAX® |
| Polyester | Polyvinyl alcohol | Polycarbonate | Polyethylene glycol | Alkyd |
| Polyamide | Polyvinyl acetate | Epoxy | Poly(lactic acid) | Fouling release |
| Acrylic | Polyvinylidene chloride | Phenoxy resin | Aramid | |
| Styrene acrylonitrile | Polyurethane | Diene elastomer | Polyimide | |
| Polyvinyl chloride | Polystyrene | Poly(oxymethylene) | Polyurethane polymer dispersion varnish | |
| Natural Materials | ||||
| Protein-based | Zein | Chitin from crustacean shells | ||
| Cellulose-based | Cotton | Cellulose | Wood pine | Viscose fiber |
| By Number of Particles [MPs L−1] | By Mass of Particles [µg L−1] | |
|---|---|---|
| Before the filter | 29 | 1.27 |
| After the filter | 3 | 0.31 |
| Removal efficiency [%] | 89.7 | 75.6 |
| BF | AF | ||
|---|---|---|---|
| Major dimension [µm] | Minimum | 6.6 | 8 |
| Maximum | 296.6 | 407.1 | |
| Median | 33.5 | 47.9 | |
| Minor dimension [µm] | Minimum | 4.2 | 4.2 |
| Maximum | 145.4 | 222.8 | |
| Median | 17.8 | 24.8 | |
| Mass [ng] | Median | 44.6 | 109.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, M.; Vianello, A.; Vollertsen, J. Removal of >10 µm Microplastic Particles from Treated Wastewater by a Disc Filter. Water 2019, 11, 1935. https://doi.org/10.3390/w11091935
Simon M, Vianello A, Vollertsen J. Removal of >10 µm Microplastic Particles from Treated Wastewater by a Disc Filter. Water. 2019; 11(9):1935. https://doi.org/10.3390/w11091935
Chicago/Turabian StyleSimon, Márta, Alvise Vianello, and Jes Vollertsen. 2019. "Removal of >10 µm Microplastic Particles from Treated Wastewater by a Disc Filter" Water 11, no. 9: 1935. https://doi.org/10.3390/w11091935
APA StyleSimon, M., Vianello, A., & Vollertsen, J. (2019). Removal of >10 µm Microplastic Particles from Treated Wastewater by a Disc Filter. Water, 11(9), 1935. https://doi.org/10.3390/w11091935