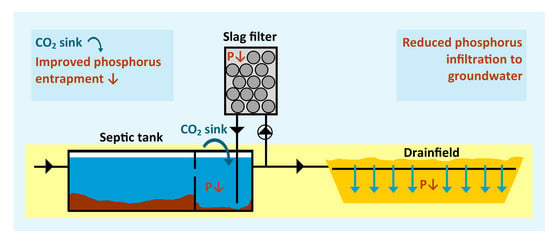
Phosphorus Removal and Carbon Dioxide Capture in a Pilot Conventional Septic System Upgraded with a Sidestream Steel Slag Filter
Abstract
:1. Introduction
- To evaluate the TP removal and CO2 capture of a pilot-scale conventional septic system, upgraded with a sidestream steel slag filter fed by the effluent of the second compartment of the septic tank, compared to a control conventional septic system without slag filter;
- To evaluate the effect of media (e.g., non-calcareous or limestone sand) on TP removal in drainfields;
- To evaluate the effect of the septic tank effluent pH on the drainfield regarding the removal of chemical oxygen demand (COD);
- To determine precipitation mechanisms and clogging risks in septic tanks and drainfields with or without a slag filter; and
- To develop design criteria for slag filters in recirculation based on a pH control strategy using modeling.
2. Materials and Methods
2.1. Septic System Pilot Tests
2.2. Slag and Sand Media
2.3. Modeling of Septic Tank with Sidestream Slag Filters
2.3.1. Production of Theoretical Equilibrium Curves of Phosphorus Mineral Phases
2.3.2. Calculation of Calcium Carbonate Saturation Index
2.3.3. Simulation of Septic Tank with a Sidestream Steel Slag Filter
2.3.4. Calculation of Carbon Dioxide Flux to Septic Tanks
3. Results and Discussion
3.1. Phosphorus Removal Performance of the Upgraded Septic Tank and Drainfield
3.2. Inorganic Carbon Fluxes in the Septic Tank
3.2.1. Modeling Calcium Carbonate Precipitation in the Septic Tank
3.2.2. Carbon Dioxide Flux into the Septic Tank
3.3. Phosphorus Removal Mechanisms in the Septic Tank
3.4. Effect of Influent Alkalinity on Steel-Slag-Filter Upgraded Septic Tank Operation and Costs
4. Conclusions and Recommendations
4.1. Recommendations for Process Design
4.2. Recommendations for Further Understanding and Improved Control
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lusk, M.G.; Toor, G.S.; Yang, Y.-Y.; Mechtensimer, S.; De, M.; Obreza, T.A. A review of the fate and transport of nitrogen, phosphorus, pathogens, and trace organic chemicals in septic systems. Crit. Rev. Environ. Sci. Technol. 2017, 47, 455–541. [Google Scholar] [CrossRef]
- EPA (United States Environmental Protection Agency). How Your Septic System Works. [WWW Document]. United States Government. 2019. Available online: https://www.epa.gov/septic/how-your-septic-system-works (accessed on 6 June 2019).
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Zamyadi, A.; MacLeod, S.L.; Fan, Y.; McQuaid, N.; Dorner, S.; Sauvé, S.; Prévost, M. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Res. 2012, 46, 1511–1523. [Google Scholar]
- Galvez-Cloutier, R.; Saminathan, S.K.M.; Boillot, C.; Triffaut-Bouchet, G.; Bourget, A.; Soumis-Dugas, G. An evaluation of several in-lake restoration techniques to improve the water quality problem (eutrophication) of Saint-Augustin lake, Quebec, Canada. Environ. Manag. 2012, 49, 1037–1053. [Google Scholar] [CrossRef]
- Penn, C.; Chagas, I.; Klimeski, A.; Lyngsie, G. A review of phosphorus removal structures: How to assess and compare their performance. Water 2017, 9, 583. [Google Scholar]
- Nilsson, C.; Renman, G.; Johansson Westholm, L.; Renman, A.; Drizo, A. Effect of organic load on phosphorus and bacteria removal from wastewater using alkaline filter materials. Water Res. 2013, 47, 6289–6297. [Google Scholar] [CrossRef] [Green Version]
- Bird, S.C.; Drizo, A. EAF steel slag filters for phosphorus removal from milk parlor effluent: The effects of solids loading, alternate feeding regimes and in-series design. Water 2010, 2, 484–499. [Google Scholar] [CrossRef] [Green Version]
- Claveau-Mallet, D.; Boutet, É.; Comeau, Y. Steel slag filter design criteria for phosphorus removal from wastewater in decentralized applications. Water Res. 2018, 143, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Shober, A.L.; Scheckel, K.G.; Penn, C.J.; Turner, K.C. Mechanisms of phosphorus removal by phosphorus sorbing materials. J. Environ. Qual. 2018, 47, 1232–1241. [Google Scholar] [PubMed]
- Hussain, S.I.; Blowes, D.W.; Ptacek, C.J.; Olding, D. Phosphorus removal from lake water using basic oxygen furnace slag: System performance and characterization of reaction products. Environ. Eng. Sci. 2014, 31, 631–642. [Google Scholar] [CrossRef]
- Zuo, M.; Renman, G.; Gustafsson, J.P.; Klysubun, W. Phosphorus removal by slag depends on its mineralogical composition: A comparative study of AOD and EAF slags. J. Water Process Eng. 2018, 25, 105–112. [Google Scholar]
- Claveau-Mallet, D.; Lida, F.; Comeau, Y. Improving phosphorus removal of conventional septic tanks by a recirculating steel slag filter. Water Qual. Res. J. Can. 2015, 50, 211–218. [Google Scholar] [CrossRef]
- Uibu, M.; Velts, O.; Kuusik, R. Developments in CO2 mineral carbonation of oil shale ash. J. Hazard. Mater. 2010, 174, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Barca, C.; Troesch, S.; Meyer, D.; Drissen, P.; Andrès, Y.; Chazarenc, F. Steel slag filters to upgrade phosphorus removal in constructed wetlands: Two years of field experiments. Environ. Sci. Technol. 2013, 47, 549–556. [Google Scholar] [PubMed]
- Kasak, K.; Mander, Ü.; Truu, J.; Truu, M.; Järveoja, J.; Maddison, M.; Teemusk, A. Alternative filter material removes phosphorus and mitigates greenhouse gas emission in horizontal subsurface flow filters for wastewater treatment. Ecol. Eng. 2015, 77, 242–249. [Google Scholar] [CrossRef]
- Bove, P.; Claveau-Mallet, D.; Boutet, É.; Lida, F.; Comeau, Y. Development and modelling of a steel slag filter effluent neutralization process with CO2-enriched air from an upstream bioprocess. Water Res. 2018, 129, 11–19. [Google Scholar] [PubMed]
- Abu-Orf, M.; Bowden, G.; Pfrang, W.; Tchobanoglous, G. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association, American Water Works Association and Water Environment Federation: Washington, DC, USA, 2012.
- Robertson, W.D.; Schiff, S.L.; Ptacek, C.J. Review of phosphate mobility and persistence in 10 septic system plumes. Groundwater 1998, 36, 1000–1010. [Google Scholar] [CrossRef]
- Charlton, S.R.; Parkhurst, D.L. Modules based on the geochemical model PHREEQC for use in scripting and programming languages. Comput. Geosci. 2011, 37, 1653–1663. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; Chapter 43 of Section A, Groundwater, Book 6, Modeling Techniques; United States Geological Survey: Denver, CO, USA, 2013.
- Claveau-Mallet, D.; Courcelles, B.; Pasquier, P.; Comeau, Y. Numerical simulations with the P-Hydroslag model to predict phosphorus removal by steel slag filters. Water Res. 2017, 126, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2, A Geochemical Assessment Model for Environmental Systems: Version 3.0 User’s Manual; Environmental Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Athens, GA, USA, 1991.
- Lizarralde, I.; Fernández-Arévalo, T.; Brouckaert, C.; Vanrolleghem, P.; Ikumi, D.S.; Ekama, G.A.; Ayesa, E.; Grau, P. A new general methodology for incorporating physico-chemical transformations into multi-phase wastewater treatment process models. Water Res. 2015, 74, 239–256. [Google Scholar] [CrossRef]
- Karabelnik, K.; Kõiv, M.; Kasak, K.; Jenssen, P.D.; Mander, Ü. High-strength greywater treatment in compact hybrid filter systems with alternative substrates. Ecol. Eng. 2012, 49, 84–92. [Google Scholar] [CrossRef]
- Robertson, W.D. Enhanced attenuation of septic system phosphate in noncalcareous sediments. Groundwter 2003, 41, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Corominas, L.; Larsen, H.F.; Flores-Alsina, X.; Vanrolleghem, P.A. Including Life Cycle Assessment for decision-making in controlling wastewater nutrient removal systems. J. Environ. Manag. 2013, 128, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.T.; Lee, Y.E.; Yoo, Y.S.; Jeong, W.; Yoon, Y.H.; Shin, D.C.; Jeong, Y. Development of a combined aerobic-anoxic and methane oxidation bioreactor system using mixed methanotrophs and biogas for wastewater denitrification. Water 2019, 11, 1377. [Google Scholar] [CrossRef] [Green Version]
- McCarty, P.L. Anaerobic treatment fundamentals. Part two, environmental requirement and control. Public Work 1964, 10, 123–126. [Google Scholar]
- Cui, W.; Liu, G.; Zeng, C.; Lu, Y.; Luo, H.; Zhang, R. Improved hydrogen production in the single-chamber microbial electrolysis cell with inhibition of methanogenesis under alkaline conditions. RSC Adv. 2019, 9, 30207–30215. [Google Scholar] [CrossRef] [Green Version]
- Vaneeckhaute, C.; Claeys, F.H.A.; Tack, F.M.G.; Meers, E.; Belia, E.; Vanrolleghem, P.A. Development, implementation, and validation of a generic nutrient recovery model (NRM) library. Environ. Model. Softw. 2018, 99, 170–209. [Google Scholar] [CrossRef]
- Valsami-Jones, E. Mineralogical controls on phosphorus recovery from wastewaters. Mineral. Mag. 2001, 65, 611–620. [Google Scholar] [CrossRef]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Robertson, W.D. Irreversible phosphorus sorption in septic system plumes? Groundwater 2007, 46, 51–60. [Google Scholar] [CrossRef]







| Reactor | Description | Volume | Influent Flow Rate | Empty Bed Contact Time |
|---|---|---|---|---|
| L | L/d | d | ||
| R1 | Three-compartment septic tank | 10,200 | 5000 to 10,000 | 1.0 to 2.0 |
| R2a | Pumping reservoir 1 | 200 | 280 | 0.7 |
| R2b | Pumping reservoir 1 | 200 | 280 | 0.7 |
| R3a | Septic tank 2 | 200 | 180 | 1.1 |
| R3b | Septic tank 2 | 200 | 180 | 1.1 |
| R4 | Steel slag filter | 180 | 45 to 135 | 1.3 to 4.0 |
| R5a | Non-calcareous drainfield | 220 | 6.8 | 32.4 |
| R5b | Non-calcareous drainfield | 220 | 6.8 | 32.4 |
| R6a | Limestone drainfield | 220 | 6.8 | 32.4 |
| R6b | Limestone drainfield | 220 | 6.8 | 32.4 |
| Parameter | Units | Value |
|---|---|---|
| COD | mg/L | 224 ± 73 |
| TSS | mg/L | 60 ± 40 |
| VSS | mg/L | 35 ± 19 |
| NH4+ | mg N/L | 20 |
| TP | mg P/L | 3.7 ± 1.2 |
| o-PO4 | mg P/L | 2.4 ± 1 |
| Ca2+ | mg/L | 136 ± 54 |
| Na+ | mg/L | 80 |
| K+ | mg/L | 9 |
| Mg2+ | mg/L | 31 |
| F− | mg/L | 14 |
| Cl− | mg/L | 131 |
| NO2− | mg N/L | <0.1 |
| NO3− | mg N/L | <0.1 |
| SO42− | mg S/L | 26 |
| pH | - | 7.2 ± 0.2 |
| Alkalinity | mg CaCO3/L | 425 ± 45 |
| Phase | Dissociation Equation | Solubility Constant |
|---|---|---|
| Hydroxyapatite | 10−46 [23] | |
| Vivianite | 10−36 [22] |
| Simulation | Methodology Using MATLAB and IPHREEQCom Modules |
|---|---|
| Influent | (1) Virtual solution simulated with the REACTION datablock using specified concentration of CaCl2, NaHCO3, KH2PO4 and K2HPO4 (2) Solution equilibrated with hydroxyapatite and calcite (saturation index of 0) using the EQUILIBRIUM_PHASES datablock |
| Slag filter effluent | (1) Influent reacted with CaO-0.4CaCl2 using the REACTION datablock (2) Solution equilibrated with hydroxyapatite and calcite using the EQUILIBRIUM_PHASES datablock (3) Iterations performed until a target pH is reached at the end of the simulation (target pH 10.5 or 11.1, representative of pH in the effluent of slag filters). |
| Septic tank effluent | (1) 100% influent mixed with abc% slag filter effluent using the MIX datablock (abc% is the recirculation ratio) (2) Solution reacted with CO2(g) using the REACTION datablock to represent biological CO2 production (3) Solution equilibrated with hydroxyapatite (saturation index of 0) and calcite (saturation index of 0.6) |
| Period | Recirculation Ratio | Mean TP Removal (%) | Mean o-PO4 Removal (%) | pH in Septic Tank Effluent | |||
|---|---|---|---|---|---|---|---|
| (d) | (%) | Control | With Slag Filter | Control | With Slag Filter | Control | With Slag Filter |
| 1 to 125 | 25 | −2 ± 10 | 17 ± 12 * | −15 ± 26 | 6 ± 23 * | 7.2 ± 0.1 | 7.4 ± 0.2 |
| 125 to 250 | 50 | −8 ± 8 | 11 ± 15 | −30 ±30 | 33 ± 26 | 7.2 ± 0.2 | 7.9 ± 0.1 |
| 250 to 275 | 75 | −7 ± 10 | 32 ± 13 | −24 ±16 | 40 ± 9 | 7.2 ± 0.3 | 8.9 ± 0.1 |
| Location in System | Estimated TP Concentration (mg P/L) | |
| Without Slag Filter | With Slag Filter | |
| Influent 1 (raw domestic wastewater) | 6 | 6 |
| Effluent of septic tank first compartment 2 | 4 | 4 |
| Effluent of septic tank second compartment 2 | 4 | 2 |
| Effluent of slag filter 2 | na | 0.1 |
| Seepage of limestone drainfield 2 | 2 | 0.5 |
| Location in System | TP Load Mass Balance (kg/d and %) | |
| Without Slag Filter | With Slag Filter | |
| Influent | 97.2 (100%) | 97.2 (100%) |
| Septic tank first compartment | 32.4 (33%) | 32.4 (33%) |
| Septic tank second compartment | 0 (0%) | 9.3 (10%) |
| Slag filter | na | 23.1 (24%) |
| Drainfield (limestone) | 32.4 (33%) | 24.3 (25%) |
| Seepage reaching groundwater | 32.4 (33%) | 8.1 (8%) |
| Sampling Location | Period | Recirculation Ratio | Mean Saturation Index | |
|---|---|---|---|---|
| (d) | (%) | Crystalline Calcite | Amorphous Calcium Carbonate | |
| Influent control | 1 to 275 | na | 0.42 | −0.92 |
| Influent with slag filter | 1 to 275 | na | 0.37 | −0.96 |
| Effluent control | 1 to 275 | na | 0.52 | −0.81 |
| Effluent with slag filter | 125 to 250 | 50 | 0.95 | −0.38 |
| Effluent with slag filter | 250 to 275 | 75 | 1.94 | 0.60 |
| Concentration in Septic Tank Headspace | CO2 Flux into Septic Tank (mol/d) | ||
|---|---|---|---|
| (ppm CO2) | With Slag Filter 50% Ratio | With Slag Filter 75% Ratio | Without Slag Filter |
| 2000 | −0.10 | 0.08 | −1.34 |
| 3000 | −0.05 | 0.13 | −1.29 |
| 5000 | 0.05 | 0.23 | −1.19 |
| 8000 | 0.20 | 0.37 | −1.04 |
| 10,000 | 0.29 | 0.47 | −0.95 |
| Expenditure Items | Scenario 1 | Scenario 2 | Scenario 3 |
|---|---|---|---|
| Capital expenditures, conventional septic system | |||
| Installation of a mainstream septic tank and a drainfield | $375 | $375 | $375 |
| Operating expenditures, conventional septic system | |||
| Septic tank sludge removal | $125 | $125 | $125 |
| Capital expenditures, slag filter upgrade * | |||
| Sidestream reactor (e.g., concrete septic tank) | - | $75 | $75 |
| ten 200-L barrels | - | $10 | $10 |
| 4 tons of 3–5 mm slag | - | $22 | $22 |
| Piping and plumbing | - | $7.50 | $7.50 |
| Pump | - | $7.50 | $7.50 |
| Operating expenditures, slag filter upgrade * | |||
| 200-L barrels | - | $20 | $100 |
| 3–5 mm slag | - | $44 | $220 |
| Piping and plumbing | - | $15 | $75 |
| Slag disposal | - | $40 | $200 |
| Total | $500 | $741 | $1217 |
| Item | Units | Scenario 1 | Scenario 2 | Scenario 3 |
|---|---|---|---|---|
| input P in one year | kg | 3.55 | 3.55 | 3.55 |
| discharged P below drainfield in one year | kg | 1.18 | 0.30 | 0.30 |
| retained P in one year | kg | 2.37 | 3.25 | 3.25 |
| Cost per removed P | $/kg P | $211 | $228 | $374 |
| Marginal cost of P retention gain by the slag filter | $/kg P | - | $272 | $808 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claveau-Mallet, D.; Seltani, H.; Comeau, Y. Phosphorus Removal and Carbon Dioxide Capture in a Pilot Conventional Septic System Upgraded with a Sidestream Steel Slag Filter. Water 2020, 12, 275. https://doi.org/10.3390/w12010275
Claveau-Mallet D, Seltani H, Comeau Y. Phosphorus Removal and Carbon Dioxide Capture in a Pilot Conventional Septic System Upgraded with a Sidestream Steel Slag Filter. Water. 2020; 12(1):275. https://doi.org/10.3390/w12010275
Chicago/Turabian StyleClaveau-Mallet, Dominique, Hatim Seltani, and Yves Comeau. 2020. "Phosphorus Removal and Carbon Dioxide Capture in a Pilot Conventional Septic System Upgraded with a Sidestream Steel Slag Filter" Water 12, no. 1: 275. https://doi.org/10.3390/w12010275
APA StyleClaveau-Mallet, D., Seltani, H., & Comeau, Y. (2020). Phosphorus Removal and Carbon Dioxide Capture in a Pilot Conventional Septic System Upgraded with a Sidestream Steel Slag Filter. Water, 12(1), 275. https://doi.org/10.3390/w12010275