The Application of Artificial Mussels in Conjunction with Transplanted Bivalves to Assess Elemental Exposure in a Platinum Mining Area
Abstract
:1. Introduction
2. Materials and Methods
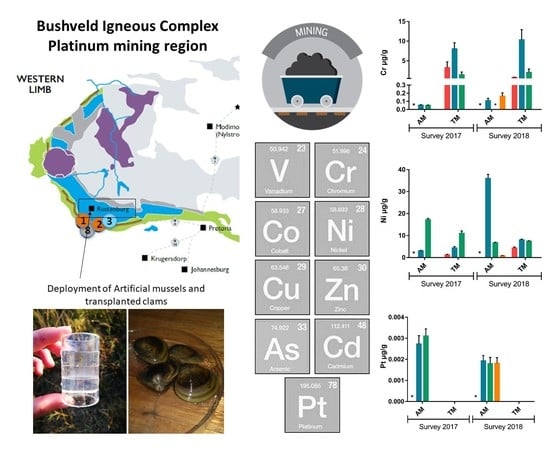
2.1. Description of the Study Area and Sampling Sites
2.2. Passive Sampling Device—Artificial Mussel
2.3. Corbicula Fluminalis Africana
2.4. Deployment of the Artificial Mussels and Clams
2.5. Retrieval of Artificial Mussels and Clams
2.6. Water Quality Parameters and Sample Collection
2.7. Element Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Metals in Water
4.2. Transplanted Organisms
4.3. Artificial Mussels
4.4. Comparison of Artificial Mussels and Transplanted Clams
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rauch, S.; Peucker-Ehrenbrink, B. Sources of Platinum Group Elements in the Environment. In Platinum Metals in the Environmen; Zereini, F., Wiseman, C.L.S., Eds.; Springer: Heidelberg, Germany, 2015; Volume 1, pp. 3–17. [Google Scholar]
- Almécija, C.; Cobelo-García, A.; Wepener, V.; Prego, R. Platinum group elements in stream sediments of mining zones: The Hex River (Bushveld Igneous Complex, South Africa). J. Afr. Earth Sci. 2017, 129, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Erasmus, J.; Malherbe, W.; Zimmermann, S.; Lorenz, A.; Nachev, M.; Wepener, V.; Sures, B.; Smit, N. Metal accumulation in riverine macroinvertebrates from a platinum mining region. Sci. Total Environ. 2019, 703, 134738. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef]
- Gupta, S.K.; Singh, J. Evaluation of mollusc as sensitive indicator of heavy metal pollution in aquatic system: A review. IIOAB J. 2011, 2, 49–57. [Google Scholar]
- Farris, J.L.; Van Hassel, J.H. Freshwater Bivalve Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Wepener, V. Application of active biomonitoring within an integrated water resources management framework in South Africa. S. Afr. J. Sci. 2008, 104, 367–373. [Google Scholar]
- Giarratano, E.; Duarte, C.A.; Amin, O.A. Biomarkers and heavy metal bioaccumulation in mussels transplanted to coastal waters of the Beagle Channel. Ecotoxicol. Environ. Saf. 2010, 73, 270–279. [Google Scholar] [CrossRef]
- Wu, R.S.S.; Lau, T.C. Polymer-ligands: A novel chemical device for monitoring heavy metals in the aquatic environment? Mar. Pollut. Bull. 1996, 32, 391–396. [Google Scholar] [CrossRef]
- Wu, R.S.S.; Lau, T.C.; Fung, W.K.M.; Ko, P.H.; Leung, K.M.Y. An ‘artificial mussel’ for monitoring heavy metals in marine environments. Environ. Pollut. 2007, 145, 104–110. [Google Scholar] [CrossRef]
- Kibria, G.; Lau, T.; Wu, R. Innovative ‘Artificial Mussels’ technology for assessing spatial and temporal distribution of metals in Goulburn–Murray catchments waterways, Victoria, Australia: Effects of climate variability (dry vs. wet years). Environ. Int. 2012, 50, 38–46. [Google Scholar] [CrossRef]
- Hossain, M.M.; Kibria, G.; Nugegoda, D.; Lau, T.; Wu, R. A Training Manual for Assessing Pollution (trace/heavy metals) in Rivers, Estuaries and Coastal waters-Using Innovative “Artificial Mussel (AM) Technology”—Bangladesh Model. Research collaboration between Scientists of the IMSF, University of Chittagong, Bangladesh, RMIT University, Australia, the City University of Hong Kong, and the University of Hong Kong; FAO: Rome, Italy, 2015; Volume 1, pp. 1–24. [Google Scholar] [CrossRef]
- Claassens, L.; Dahms, S.; Van Vuren, J.; Greenfield, R. Artificial mussels as indicators of metal pollution in freshwater systems: A field evaluation in the Koekemoer Spruit, South Africa. Ecol. Indic. 2016, 60, 940–946. [Google Scholar] [CrossRef]
- Leung, K.M.Y.; Furness, R.W.; Svavarsson, J.; Lau, T.C.; Wu, R.S.S. Field validation, in Scotland and Iceland, of the artificial mussel for monitoring trace metals in temperate seas. Mar. Pollut. Bull. 2008, 57, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Degger, N.; Wepener, V.; Richardson, B.J.; Wu, R.S.S. Application of artificial mussels (AMs) under South African marine conditions: A validation study. Mar. Pollut. Bull. 2011, 63, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Fatoki, O.S. Impact of Platinum Group Element Emissions from Mining and Production Activities. In Platinum Metals in the Environment; Zereini, F., Wiseman, C.L.S., Eds.; Springer: Heidelberg, Germany, 2015; pp. 19–29. [Google Scholar] [CrossRef]
- Wittmann, G.; Förstner, U. Heavy metal enrichment in mine drainage: 1. The Rustenburg platinum mining area. Science 1976, 72, 242–246. [Google Scholar]
- Roux, H. Status of the yellowfish populations & River health Programme in NW Province. In Proceedings of the 15th Yellowfish Working Group Conference, Mpumalanga, South Africa, 18–20 February 2011. [Google Scholar]
- Mogakabe, D.; Van Ginkel, C. The Water Quality of the Bospoort Dam; Internal Report No. N/0000/00/DEQ/0108; Department of Water Affairs and Forestry: Pretoria, South Africa, 2008.
- Dahms-Verster, S.; Baker, N.; Greenfield, R. A multivariate examination of ‘artificial mussels’ in conjunction with spot water tests in freshwater ecosystems. Environ. Monit. Assess. 2018, 190, 427. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.J.; Erasmus, J.H.; Labuschagne, M.; Grabner, D.; Nachev, M.; Zimmermann, S.; Wepener, V.; Smit, N.; Sures, B. Bioaccumulation and metal-associated biomarker responses in a freshwater mussel, Dreissena polymorpha, following short-term platinum exposure. Environ. Pollut. 2019, 246, 69–78. [Google Scholar] [CrossRef]
- Zimmermann, S.; Menzel, C.M.; Berner, Z.; Eckhardt, J.-D.; Stüben, D.; Alt, F.; Messerschmidt, J.; Taraschewski, H.; Sures, B. Trace analysis of platinum in biological samples: A comparison between sector field ICP-MS and adsorptive cathodic stripping voltammetry following different digestion procedures. Anal. Chim. Acta 2001, 439, 203–209. [Google Scholar] [CrossRef]
- Degger, N.; Chiu, J.M.Y.; Po, B.H.K.; Tse, A.C.K.; Zheng, G.J.; Zhao, D.-M.; Xu, D.; Cheng, Y.-S.; Wang, X.-H.; Liu, W.-H.; et al. Heavy metal contamination along the China coastline: A comprehensive study using Artificial Mussels and native mussels. Environ. Manag. 2016, 180, 238–246. [Google Scholar] [CrossRef]
- Kushner, E. On determining the statistical parameters for pollution concentration from a truncated data set. Atmos. Environ. 1976, 10, 975–979. [Google Scholar] [CrossRef]
- Cawthorn, R.G. The platinum group element deposits of the Bushveld Complex in South Africa. Platin. Met. Rev. 2010, 54, 205–215. [Google Scholar] [CrossRef]
- Thorsen, S. Past Weather in Rustenburg, South Africa—May 2017. Available online: https://www.timeanddate.com/weather/south-africa/rustenburg/historic?month=5&year=2017 (accessed on 1 October 2018).
- Prathumratana, L.; Sthiannopkao, S.; Kim, K.W. The relationship of climatic and hydrological parameters to surface water quality in the lower Mekong River. Environ. Int. 2008, 34, 860–866. [Google Scholar] [CrossRef]
- Department of Water Affairs and Forestry (DWAF). South African Water Quality Guidelines (Second Edition). Volume 1: Domestic Use; DWAF: Pretoria, South Africa, 1996.
- Department of Water Affairs and Forestry (DWAF). South African Water Quality Guidelines. Volume 7: Aquatic Ecosystems; DWAF: Pretoria, South Africa, 1996.
- Gerber, R.; Smit, N.J.; van Vuren, J.H.; Wepener, V. Metal concentrations in Hydrocynus vittatus (Castelnau 1861) populations from a premier conservation area: Relationships with environmental concentrations. Ecotoxicol. Environ. Saf. 2016, 129, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.; Wepener, V.; de Kock, K.; Wolmarans, C. Metallothionein Induction as Indicator of Low Level Metal Exposure to Aquatic Macroinvertebrates from a Relatively Unimpacted River System in South Africa. Bull. Environ. Contam. Toxicol. 2017, 99, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Hoppstock, K.; Sures, B. Platinum-Group Metals. In Elements and Their Compounds in the Environment: Occurrence, Analysis and Biological Relevance, 2nd ed.; Merian, E., Anke, M., Ihnat, M., Stoeppler, M., Eds.; WILEY-VCH Verlag GMBH & Co. KGaA: Weinheim, Germany, 2004; pp. 1047–1086. [Google Scholar]
- Zimmermann, S.; Alt, F.; Messerschmidt, J.; von Bohlen, A.; Taraschewski, H.; Sures, B. Biological availability of traffic-related platinum-group elements (palladium, platinum, and rhodium) and other metals to the zebra mussel (Dreissena polymorpha) in water containing road dust. Environ. Toxicol. Chem. 2002, 21, 2713–2718. [Google Scholar] [CrossRef] [PubMed]
- Hoppstock, K.; Alt, F. Voltammetric determination of ultratrace platinum and rhodium in biological and environmental samples. In Anthropogenic Platinum-Group Element Emissions; Fathi, Z., Friedrich, A., Eds.; Springer: Heidelberg, Germany, 2000; pp. 145–152. [Google Scholar] [CrossRef]
- Moldovan, M.; Gomez, M.M.; Palacios, M.A. On-line preconcentration of palladium on alumina microcolumns and determination in urban waters by inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2003, 478, 209–217. [Google Scholar] [CrossRef]
- Monticelli, D.; Carugati, G.; Castelletti, A.; Recchia, S.; Dossi, C. Design and development of a low cost, high performance UV digester prototype: Application to the determination of trace elements by stripping voltammetry. Microchemical 2010, 95, 158–163. [Google Scholar] [CrossRef]
- Cobelo-García, A.; López-Sánchez, D.E.; Almécija, C.; Santos-Echeandía, J. Behavior of platinum during estuarine mixing (Pontevedra Ria, NW Iberian Peninsula). Mar. Chem. 2013, 150, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Ruchter, N.; Sures, B. Distribution of platinum and other traffic related metals in sediments and clams (Corbicula sp.). Water Res. 2015, 70, 313–324. [Google Scholar] [CrossRef]
- van Heerden, D.; van Rensburg, P.J.; Nikinmaa, M.; Vosloo, A. Gill damage, metallothionein gene expression and metal accumulation in Tilapia sparrmanii from selected field sites at Rustenburg and Potchefstroom, South Africa. Afr. J. Aquat. Sci. 2006, 31, 89–98. [Google Scholar] [CrossRef]
- Ruchter, N.; Zimmermann, S.; Sures, B. Field studies on PGE in aquatic ecosystems. In Platinum Metals in the Environment; Zereini, F., Wiseman, C.L.S., Eds.; Springer: Heidelberg, Germany, 2015; pp. 351–360. [Google Scholar] [CrossRef]
- Greenfield, R.; Brink, K.; Degger, N.; Wepener, V. The usefulness of transplantation studies in monitoring of metals in the marine environment: South African experience. Mar. Pollut. Bull. 2014, 85, 566–573. [Google Scholar] [CrossRef]
- Genç, T.O.; Po, B.H.; Yılmaz, F.; Lau, T.C.; Wu, R.S.; Chiu, J.M. Differences in metal profiles revealed by native mussels and artificial mussels in Sarıçay Stream, Turkey: Implications for pollution monitoring. Mar. Freshw. Res. 2018, 69, 1372–1378. [Google Scholar] [CrossRef]
- Morquecho, R.; Pitt, R. Stormwater heavy metal particulate associations. Proc. Water Environ. Fed. 2003, 2003, 774–803. [Google Scholar] [CrossRef]
- Nor, Y.M. The absorption of metal ions by Eichhornia crassipes. Chem. Speciat. Bioavailab. 1990, 2, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Shirinpur-Valadi, A.; Hatamzadeh, A.; Sedaghathoor, S. Study of the accumulation of contaminants by Cyperus alternifolius, Lemna minor, Eichhornia crassipes, and Canna generalis in some contaminated aquatic environments. Environ. Sci. Pollut. Res. Int. 2019, 26, 21340–21350. [Google Scholar] [CrossRef] [PubMed]









| Metal Analysed | Detection Limit for Water (µg/L) | Detection Limit for Artificial Mussels (µg/g) | Detection Limit for Transplanted Clams (µg/g) |
|---|---|---|---|
| As | 0.203 | 0.0056 | 0.0169 |
| Cd | 0.022 | 0.0004 | 0.0018 |
| Co | 0.112 | 0.003 | 0.0099 |
| Cr | 0.151 | 0.039 | 0.130 |
| Ni | 0.463 | 0.011 | 0.0386 |
| Pb | 0.0237 | 0.0003 | 0.0020 |
| Pt | 0.0107 | 0.00015 | 0.0009 |
| V | 0.96 | 0.025 | 0.0798 |
| Zn | 17.7 | 0.053 | 1.48 |
| Site | pH | Temp (°C) | EC (μS/cm) | TDS (mg/L) | DO (mg/L) | DO (%) |
|---|---|---|---|---|---|---|
| Survey 2017 | ||||||
| Olifantsnek Dam | 8.21 | 19.3 | 173.8 | 119.8 | 10.12 | 103.3 |
| Bospoort Dam | 8.46 | 19.45 | 910.5 | 570 | 9.69 | 81.4 |
| Survey 2018 | ||||||
| Olifantsnek Dam | 8.20 | 24.3 | 210 | 147 | 5.5 | 62.1 |
| Bospoort Dam | 7.65 | 28.3 | 1160 | 819 | 2.94 | 34.3 |
| Pollution control dam | 8.00 | 25.6 | 1775 | 1244 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labuschagne, M.; Wepener, V.; Nachev, M.; Zimmermann, S.; Sures, B.; Smit, N.J. The Application of Artificial Mussels in Conjunction with Transplanted Bivalves to Assess Elemental Exposure in a Platinum Mining Area. Water 2020, 12, 32. https://doi.org/10.3390/w12010032
Labuschagne M, Wepener V, Nachev M, Zimmermann S, Sures B, Smit NJ. The Application of Artificial Mussels in Conjunction with Transplanted Bivalves to Assess Elemental Exposure in a Platinum Mining Area. Water. 2020; 12(1):32. https://doi.org/10.3390/w12010032
Chicago/Turabian StyleLabuschagne, Marelize, Victor Wepener, Milen Nachev, Sonja Zimmermann, Bernd Sures, and Nico J. Smit. 2020. "The Application of Artificial Mussels in Conjunction with Transplanted Bivalves to Assess Elemental Exposure in a Platinum Mining Area" Water 12, no. 1: 32. https://doi.org/10.3390/w12010032
APA StyleLabuschagne, M., Wepener, V., Nachev, M., Zimmermann, S., Sures, B., & Smit, N. J. (2020). The Application of Artificial Mussels in Conjunction with Transplanted Bivalves to Assess Elemental Exposure in a Platinum Mining Area. Water, 12(1), 32. https://doi.org/10.3390/w12010032