Occurrence of Free Amino Acids in the Source Waters of Zhejiang Province, China, and Their Removal and Transformation in Drinking Water Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Derivatization Procedure
2.3. Conditions of GC-MS
2.4. DBP Formation Potential of FAAs
2.5. Sample Information
3. Results and Discussion
3.1. Principle of the Method
3.2. Calibration Curves
3.3. Reliability Assessment of FAA Derivatization
3.4. The Occurrence of FAAs in Source and Treated Water Samples
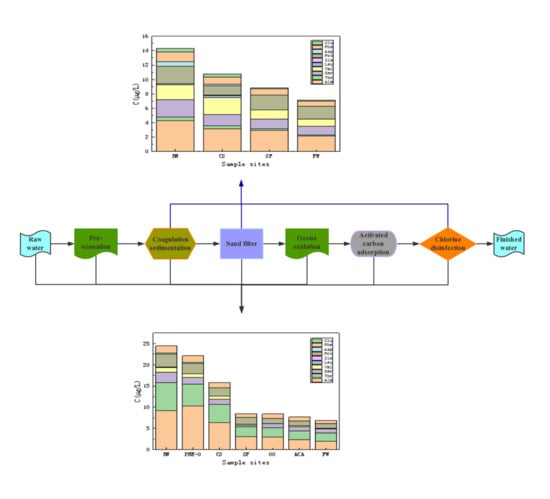
3.5. The Degradation of FAAs in Different Water Treatment Plants
3.6. DBP Formation Potential of Typical FAAs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Westerhoff, P.; Mash, H. Dissolved organic nitrogen in drinking water supplies: A review. J. Water Supply Res. Technol. AQUA 2002, 51, 415–448. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Gao, N.; Krasner, S.W.; Templeton, M.R.; Yin, D. Formation of halogenated c-, n-dbps from chlor(am)ination and uv irradiation of tyrosine in drinking water. Environ. Pollut. 2012, 161, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, T.; Templeton, M.R.; Graham, N. Precursors of nitrogenous disinfection by-products in drinking water—A critical review and analysis. J. Hazard. Mater. 2012, 235, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-H.; Gao, N.-Y.; Deng, Y.; Dong, B.-Z. Formation of chloroform during chlorination of alanine in drinking water. Chemosphere 2009, 77, 1346–1351. [Google Scholar] [CrossRef]
- Yao, D.; Chu, W.; Bond, T.; Ding, S.; Chen, S. Impact of clo2 pre-oxidation on the formation of cx3r-type dbps from tyrosine-based amino acid precursors during chlorination and chloramination. Chemosphere 2018, 196, 25–34. [Google Scholar] [CrossRef]
- Muellner, M.G.; Wagner, E.D.; McCalla, K.; Richardson, S.D.; Woo, Y.-T.; Plewa, M.J. Haloacetonitriles vs. Regulated haloacetic acids: Are nitrogen-containing dbps more toxic? Environ. Sci. Technol. 2007, 41, 645–651. [Google Scholar] [CrossRef]
- Yang, L.; Kim, D.; Uzun, H.; Karanfil, T.; Hur, J. Assessing trihalomethanes (thms) and n-nitrosodimethylamine (ndma) formation potentials in drinking water treatment plants using fluorescence spectroscopy and parallel factor analysis. Chemosphere 2015, 121, 84–91. [Google Scholar] [CrossRef]
- Yang, X.; Shen, Q.; Guo, W.; Peng, J.; Liang, Y. Precursors and nitrogen origins of trichloronitromethane and dichloroacetonitrile during chlorination/chloramination. Chemosphere 2012, 88, 25–32. [Google Scholar] [CrossRef]
- Yu, Y.; Reckhow, D.A. Kinetic analysis of haloacetonitrile stability in drinking waters. Environ. Sci. Technol. 2015, 49, 11028–11036. [Google Scholar] [CrossRef]
- Brosillon, S.; Lemasle, M.; Renault, E.; Tozza, D.; Heim, V.; Laplanche, A. Analysis and occurrence of odorous disinfection by-products from chlorination of amino acids in three different drinking water treatment plants and corresponding distribution networks. Chemosphere 2009, 77, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- How, Z.T.; Busetti, F.; Linge, K.L.; Kristiana, I.; Joll, C.A.; Charrois, J.W. Analysis of free amino acids in natural waters by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1370, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Z.; MacMillan, D.W.C. Decarboxylative arylation of α-amino acids via photoredox catalysis: A one-step conversion of biomass to drug pharmacophore. J. Am. Chem. Soc. 2014, 136, 5257–5260. [Google Scholar] [CrossRef] [PubMed]
- Dotson, A.; Westerhoff, P.; Krasner, S.W. Nitrogen enriched dissolved organic matter (dom) isolates and their affinity to form emerging disinfection by-products. Water Sci. Technol. 2009, 60, 135–143. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, K.Y.; Hamm, S.-Y.; Kim, M.; Kim, H.K.; Oh, J.-E. Occurrence and distribution of pharmaceutical and personal care products, artificial sweeteners, and pesticides in groundwater from an agricultural area in korea. Sci. Total Environ. 2019, 659, 168–176. [Google Scholar] [CrossRef]
- Punturat, V.; Huang, K.-L. Degradation of acesulfame in aqueous solutions by electro-oxidation. J. Taiwan Inst. Chem. Eng. 2016, 63, 286–294. [Google Scholar] [CrossRef]
- Scheurer, M.; Schmutz, B.; Happel, O.; Brauch, H.-J.; Wülser, R.; Storck, F.R. Transformation of the artificial sweetener acesulfame by uv light. Sci. Total Environ. 2014, 481, 425–432. [Google Scholar] [CrossRef]
- Soh, L.; Connors, K.A.; Brooks, B.W.; Zimmerman, J. Fate of sucralose through environmental and water treatment processes and impact on plant indicator species. Environ. Sci. Technol. 2011, 45, 1363–1369. [Google Scholar] [CrossRef]
- Toth, J.E.; Rickman, K.A.; Venter, A.R.; Kiddle, J.J.; Mezyk, S.P. Reaction kinetics and efficiencies for the hydroxyl and sulfate radical based oxidation of artificial sweeteners in water. J. Phys. Chem. A 2012, 116, 9819–9824. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Jia, S.; Wu, G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by hplc with fluorescence detection. J. Chromatogr. B 2014, 964, 116–127. [Google Scholar] [CrossRef]
- De Vera, G.A.; Stalter, D.; Gernjak, W.; Weinberg, H.S.; Keller, J.; Farré, M.J. Towards reducing dbp formation potential of drinking water by favouring direct ozone over hydroxyl radical reactions during ozonation. Water Res. 2015, 87, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, L.; Liu, G.; Hongbing, X.; Luo, R. Quantification of the nonpolar amino acids in amniotic fluid of congenital malformation by gas chromatography mass spectrometry (gc-ms) derivatization method. Basic Clin. Med. 2017, 37, 1004–1009. [Google Scholar]
- Chu, W.; Li, C.; Gao, N.; Templeton, M.R.; Zhang, Y. Terminating pre-ozonation prior to biological activated carbon filtration results in increased formation of nitrogenous disinfection by-products upon subsequent chlorination. Chemosphere 2015, 121, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Deng, C.; Zhang, P.; Hu, Y. Simultaneous quantitative analysis of amino acids in blood or of phenylpyruvic and p-hydroyphenylacetic acid in urine of newborns by gc-ms. J. Fudan Univ. (Nat. Sci.) 2002, 41, 413–418. [Google Scholar]
- Hu, J.; Song, H.; Karanfil, T. Comparative analysis of halonitromethane and trihalomethane formation and speciation in drinking water: The effects of disinfectants, ph, bromide, and nitrite. Environ. Sci. Technol. 2010, 44, 794–799. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, G.; Gao, G.; Qin, B. Spatialdistribution ofdissloved aminoacidsin LakeTaihu, China. Acta Ecol. Sin. 2013, 33. [Google Scholar] [CrossRef]
- Chinn, R.; Barrett, S.E. Occurrence of amino acids in two drinking water sources. In Natural Organic Matter and Disinfection by-Products; American Chemical Society: Washington, DC, USA, 2000; Volume 761, pp. 96–108. [Google Scholar]
- Hong, H.C.; Wong, M.H.; Liang, Y. Amino acids as precursors of trihalomethane and haloacetic acid formation during chlorination. Arch. Environ. Contam. Toxicol. 2009, 56, 638–645. [Google Scholar] [CrossRef]
- Gerrity, D.; Gamage, S.; Holady, J.C.; Mawhinney, D.B.; Quiñones, O.; Trenholm, R.A.; Snyder, S.A. Pilot-scale evaluation of ozone and biological activated carbon for trace organic contaminant mitigation and disinfection. Water Res. 2011, 45, 2155–2165. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, M.; Zou, B.; Li, N. Mechanism of chlorination of threonine disinfection by-product trichloroacetone in drinking water. J. Chem. Ind. Eng. 2016, 67, 3010–3015. [Google Scholar]
- Ding, S.; Wang, F.; Chu, W.; Cao, Z.; Pan, Y.; Gao, N. Rapid degradation of brominated and iodinated haloacetamides with sulfite in drinking water: Degradation kinetics and mechanisms. Water Res. 2018, 143, 325–333. [Google Scholar] [CrossRef]
- Ma, X.; Deng, J.; Feng, J.; Shanaiah, N.; Smiley, E.; Dietrich, A.M. Identification and characterization of phenylacetonitrile as a nitrogenous disinfection byproduct derived from chlorination of phenylalanine in drinking water. Water Res. 2016, 102, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, N.; Chu, W.; Bond, T.; Wei, X. Comparison of thms and hans formation potential from the chlorination of free and combined histidine and glycine. Chem. Eng. J. 2017, 307, 487–495. [Google Scholar] [CrossRef]
- Chu, W.; Gao, N. Classification and toxicological evaluation of newfound nitrogenous disinfection byproducts(N-DBPs) in drinking water. Mod. Chem. Ind. 2009, 29, 86–89. [Google Scholar]





| Water Quality Samples | TOC mg/L | Conductivity μs/cm | pH | |
|---|---|---|---|---|
| ① | RW | 111.6–121.4 | 1500–1810 | 8.0–8.2 |
| FW | 2.3–3.1 | 710–1310 | 6.9–7.9 | |
| ② | RW | 1.9–4.2 | 101–120 | 7.4–7.8 |
| FW | 1.4–2.5 | 98–118 | 7.2–7.5 | |
| ③ | RW | 2.5–3.9 | 88–130 | 7.4–7.9 |
| FW | 1.0–2.7 | 80–104 | 7.2–7.5 | |
| ④ | RW | 1.9–4.2 | 110–129 | 7.2–7.7 |
| FW | 1.2–2.6 | 96–107 | 7.1–7.4 | |
| ⑤ | RW | 2.7–4.2 | 117–222 | 7.4–7.8 |
| FW | 2.1–3.4 | 121–189 | 7.3–7.8 | |
| ⑥ | RW | 2.2–5.9 | 99–336 | 7.1–7.5 |
| FW | 1.3–1.6 | 105–110 | 7.0–7.2 | |
| ⑦ | RW | 3.8–8.7 | 314–452 | 7.5–7.8 |
| FW | 1.6–3.2 | 301–455 | 7.1–7.2 | |
| Acronym | Glossary Explaining |
|---|---|
| FAAs | free amino acids |
| DBPs | disinfection by-products |
| N-DBPs | nitrogenous disinfection by-products |
| C-DBPs | carbon disinfection by-products |
| THMs | trihalomethanes |
| HAAs | haloaceticacids |
| HANs | haloacetonitriles |
| HAcAms | haloacetamides |
| HNMs | halonitromethanes |
| TCM | trichloromethane |
| DCAN | dichloroacetonitrile |
| DCAA | dichloroacetic acid |
| TCAA | trichloroacetic acid |
| TCNM | trichloronitromethane |
| Ala | alanine |
| Thr | threonine |
| Ser | serine |
| Val | valine |
| Leu | leucine |
| Ileu | isoleucine |
| Pro | proline |
| Asp | aspartic |
| Phe | phenylalanine |
| Glu | glutamic acid |
| Try | tryptophan |
| Gly | glycine |
| RW | raw water |
| FW | finished water |
| SCX | strong cation exchange filler |
| JX, NXQ, QT, XF, CX, and TH | six waterworks in Zehjiang province |
| Free Amino Acid | Derivative Molecular Ion (m/z) | Base Peak (m/z) | Characteristic Ions (m/z) | Retention Time (min) | Limit of Detection (μg/L) |
|---|---|---|---|---|---|
| Ala | 241 | 140 | 41, 57, 69,140 | 9.43 | 5.6 |
| Thr | 271 | 57 | 41, 57, 69, 153 | 10.13 | 10.8 |
| Ser | 353 | 57 | 57, 69, 41, 139 | 10.30 | 13.9 |
| Val | 269 | 168 | 168, 55, 41,57, 168 | 10.53 | 5.1 |
| Leu | 283 | 69 | 41, 182, 140, 57, 69 | 11.21 | 2.9 |
| Ileu | 283 | 69 | 41, 57, 152, 69, 182 | 11.32 | 3.2 |
| Pro | 267 | 166 | 41, 69, 96, 166 | 12.47 | 2.3 |
| Asp | 341 | 57 | 41, 240, 139, 184, 57 | 14.18 | 3.9 |
| Phe | 317 | 91 | 41, 148, 204, 91 | 14.36 | 2.4 |
| Glu | 355 | 180 | 41, 57, 180, 152, 198, 254 | 15.20 | 3.4 |
| FAA | Pure Water (%) | Tap Water (%) | Raw Water (%) | Mean (s.d) (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 5 | 10 | 20 | 5 | 10 | 20 | ||
| (μg/L) | (μg/L) | (μg/L) | ||||||||
| Ala | 84.2 ± 1.2 | 82.6 ± 2.3 | 84.3 ± 0.9 | 86.6 ± 4.5 | 87.6 ± 2.5 | 85.4 ± 1.8 | 80.4 ± 3.6 | 82.6 ± 1.3 | 89.4 ± 3.4 | 84.8 (2.8) |
| Thr | 72.3 ± 2.5 | 74.2 ± 1.6 | 77.3 ± 2.3 | 67.6 ± 3.2 | 65.8 ± 3.4 | 65.4 ± 4.6 | 70.3 ± 2.4 | 72.8 ± 2.6 | 69.0 ± 2.9 | 74.5 (4.0) |
| Ser | 89.7 ± 1.9 | 95.6 ± 2.8 | 92.2 ± 1.8 | 71.4 ± 3.8 | 73.9 ± 1.8 | 72.3 ± 5.1 | 63.5 ± 3.1 | 70.9 ± 1.4 | 76.6 ± 1.2 | 78.5 (11.2) |
| Val | 86.5 ± 1.8 | 84.4 ± 3.1 | 87.6 ± 1.7 | 94.3 ± 1.6 | 90.6 ± 1.3 | 92.3 ± 3.8 | 91.3 ± 1.9 | 92.6 ± 3.7 | 90.0 ± 4.7 | 90.0 (3.2) |
| Leu | 83.2 ± 2.7 | 80.4 ± 4.7 | 82.6 ± 3.5 | 82.3 ± 2.2 | 79.0 ± 2.6 | 86.8 ± 4.5 | 80.2 ± 1.6 | 85.6 ± 4.2 | 83.1 ± 5.3 | 82.6 (2.5) |
| Ileu | 85.4 ± 3.6 | 89.6 ± 2.9 | 82.4 ± 4.7 | 87.8 ± 3.4 | 86.6 ± 3.7 | 92.4 ± 3.2 | 87.7 ± 2.8 | 85.6 ± 3.8 | 88.4 ± 2.7 | 87.3 (2.8) |
| Pro | 87.6 ± 5.2 | 85.5 ± 4.1 | 80.4 ± 2.6 | 85.6 ± 1.1 | 87.5 ± 2.9 | 76.5 ± 2.8 | 87.1 ± 3.4 | 89.4 ± 4.9 | 90.4 ± 3.8 | 85.6 (4.4) |
| Asp | 73.5 ± 1.3 | 71.1 ± 3.5 | 75.6 ± 1.5 | 72.3 ± 3.6 | 73.7 ± 1.2 | 81.5 ± 1.6 | 72.3 ± 5.4 | 72.9 ± 4.6 | 76.5 ± 1.6 | 74.4 (3.2) |
| Phe | 89.4 ± 3.4 | 90.2 ± 2.4 | 92.4 ± 1.2 | 79.1 ± 1.5 | 83.8 ± 0.8 | 75.4 ± 1.9 | 83.1 ± 3.2 | 87.8 ± 2.5 | 84.5 ± 2.9 | 85.1 (5.5) |
| Glu | 79.1 ± 4.1 | 78.8 ± 1.3 | 76.8 ± 4.8 | 72.8 ± 2.4 | 73.6 ± 5.3 | 75.8 ± 3.7 | 75.2 ± 1.4 | 73.5 ± 1.9 | 76.7 ± 3.8 | 75.8 (2.3) |
| Mean FAAs | 81.3 | 83.2 | 83.2 | 80.0 | 80.2 | 80.4 | 79.1 | 81.4 | 82.5 | 81.5 (1.6) |
| s.d. FAAs | 6.2 | 7.5 | 6.0 | 8.7 | 8.2 | 8.0 | 8.7 | 8.1 | 7.4 | |
| Waterworks | JX Waterworks (μg/L) | NXQ Waterworks (μg/L) | QTWaterworks (μg/L) | XF Waterworks (μg/L) | CXWaterworks (μg/L) | THWaterworks (μg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Process | Surface Water-Coagulation, Flocculation, Sedimentation, Filtration, Chlorination | Surface Water-Coagulation, Flocculation, Sedimentation, Ozonation-Bioactive Carbon, Filtration, Chlorination | Surface Water-Flocculation, Sedimentation, Filtration, Micromembrane, Chlorination | Surface Water-Coagulation, Flocculation, Sedimentation, Filtration, Chlorination | Surface Water-Coagulation, Flocculation, Sedimentation, Filtration, Chlorination | Surface Water-Preozonation, Coagulation, Flocculation, Sedimentation, Ozonation, Active Carbon Filtration, Chlorination | |||||||
| Free Amino Acid | Date | Raw Water | Finished Water | Raw Water | Finished Water | Raw Water | Finished Water | Raw Water | Finished Water | Raw Water | Finished Water | Raw Water | Finished Water |
| Ala | ① | 7.39 | 7.49 | 14.73 | 10.10 | 9.27 | 4.68 | 11.36 | 7.78 | 6.34 | 4.33 | 10.23 | 1.56 |
| ② | 6.61 | 1.48 | 14.36 | 12.70 | 8.02 | 3.56 | 14.10 | 6.15 | 6.73 | 3.28 | 8.43 | 1.62 | |
| ③ | 6.93 | 1.77 | 5.00 | 2.79 | 8.21 | 1.73 | 4.27 | 2.19 | 0.57 | 0.45 | ND | ND | |
| ④ | 5.81 | -- | 4.73 | -- | 5.52 | -- | -- | -- | 4.27 | 2.14 | 9.23 | 1.97 | |
| Thr | ① | 1.44 | 1.16 | 2.39 | 0.93 | 0.87 | ND | 1.97 | 1.79 | 0.93 | 0.50 | 1.02 | 0.85 |
| ② | 1.69 | 0.30 | 1.41 | 0.52 | 0.47 | ND | 0.57 | 0.20 | 1.23 | 0.64 | 0.55 | ND | |
| ③ | 0.92 | 0.34 | 0.43 | 0.55 | 0.48 | 1.49 | 0.72 | 0.21 | -- | -- | -- | -- | |
| ④ | 1.07 | -- | 1.24 | -- | 1.30 | -- | -- | -- | 0.48 | 0.12 | 6.58 | 2.01 | |
| Ser | ① | 7.84 | 7.26 | ND | ND | 3.81 | 2.15 | ND | ND | 3.45 | 3.01 | 3.71 | 0.98 |
| ② | 5.19 | 3.83 | 1.78 | 1.12 | 1.21 | 0.41 | 1.67 | 0.52 | 5.09 | 2.47 | 2.06 | ND | |
| ③ | 1.25 | 1.01 | 1.83 | 1.11 | 1.26 | 0.52 | 1.70 | 0.62 | 1.80 | 3.23 | 0.60 | 0.88 | |
| ④ | 5.00 | -- | 3.58 | -- | 3.94 | -- | -- | -- | 2.46 | 1.25 | 2.38 | 0.96 | |
| Val | ① | 3.19 | 1.40 | 6.86 | 1.64 | ND | ND | 6.64 | 2.00 | 1.31 | 0.71 | 1.46 | ND |
| ② | 3.23 | 1.24 | 5.97 | 2.36 | 0.40 | 0.23 | 6.20 | 3.14 | 1.39 | 0.21 | 0.55 | ND | |
| ③ | 0.24 | 0.23 | 0.74 | 0.56 | 0.50 | 0.22 | 0.63 | ND | 0.99 | 0.85 | 0.49 | 0.24 | |
| ④ | 1.77 | -- | 1.64 | -- | 1.52 | -- | -- | -- | 2.04 | 0.98 | 1.13 | 0.12 | |
| Leu | ① | 2.20 | 1.80 | 3.07 | 1.89 | 3.00 | 1.90 | 4.10 | 2.02 | 0.13 | 0.11 | 0.17 | 0.14 |
| ② | 3.48 | 1.56 | 4.16 | 1.29 | 4.01 | 1.72 | 3.90 | 2.40 | 0.47 | 0.12 | ND | ND | |
| ③ | 0.56 | 0.37 | 0.55 | 0.48 | 0.43 | 0.18 | 0.34 | 0.47 | ND | ND | ND | ND | |
| ④ | 1.77 | -- | 1.38 | -- | 2.13 | -- | -- | -- | 0.19 | ND | 0.12 | ND | |
| Ile | ① | ND | ND | ND | ND | ND | ND | ND | ND | 1.54 | 0.57 | ND | ND |
| ② | ND | ND | ND | ND | ND | ND | ND | ND | 1.34 | ND | ND | ND | |
| ③ | 1.54 | ND | 0.05 | 0.02 | ND | ND | 0.08 | ND | ND | 0.47 | 0.21 | 0.62 | |
| ④ | 1.08 | -- | 1.65 | -- | 1.70 | -- | -- | -- | ND | ND | ND | ND | |
| Pro | ① | ND | ND | 8.06 | 1.80 | 1.90 | 0.84 | 4.15 | ND | 1.80 | 1.60 | 2.48 | ND |
| ② | 1.20 | 0.67 | 6.19 | 0.96 | 0.98 | 0.64 | 1.02 | 0.60 | ND | ND | 1.84 | 0.24 | |
| ③ | 1.39 | 0.77 | 1.30 | 1.01 | 1.05 | 0.75 | 1.03 | 0.63 | ND | ND | ND | ND | |
| ④ | 3.03 | -- | 2.85 | -- | 3.54 | -- | -- | -- | 2.41 | 1.75 | 3.09 | 1.04 | |
| Asp | ① | 2.11 | 1.24 | 4.73 | 1.39 | 2.12 | ND | 3.42 | 1.91 | ND | 0.90 | ND | ND |
| ② | 2.52 | 0.69 | 2.91 | 0.74 | 0.65 | 0.42 | 0.94 | ND | 2.18 | 0.88 | ND | 0.49 | |
| ③ | 3.92 | 0.80 | 1.62 | 0.89 | 0.69 | 0.49 | 1.02 | ND | ND | ND | ND | ND | |
| ④ | ND | -- | ND | -- | ND | -- | -- | -- | 0.62 | ND | 0.27 | ND | |
| Phe | ① | 1.13 | ND | 2.26 | 1.28 | 1.22 | 0.44 | 1.76 | 1.16 | 1.00 | 0.41 | 1.59 | ND |
| ② | 3.01 | ND | 1.59 | ND | 0.46 | 0.31 | 1.59 | 0.41 | 0.89 | 1.07 | ND | 0.30 | |
| ③ | 1.81 | ND | 1.45 | 0.59 | ND | 0.69 | ND | ND | 5.19 | 5.19 | 5.14 | 4.27 | |
| ④ | ND | -- | 1.50 | -- | 1.58 | -- | -- | -- | 1.37 | 0.78 | 1.63 | 0.74 | |
| Glu | ① | ND | ND | 3.83 | 0.72 | 1.00 | 0.29 | 2.00 | 1.43 | ND | 0.66 | ND | 0.69 |
| ② | 0.41 | 0.23 | 2.20 | 0.85 | 1.30 | ND | 3.41 | ND | 0.44 | 0.12 | ND | ND | |
| ③ | 4.82 | 0.42 | 4.59 | 0.23 | ND | 0.08 | ND | 0.13 | ND | ND | ND | ND | |
| ④ | ND | -- | ND | -- | ND | -- | -- | -- | 0.51 | 0.12 | ND | ND | |
| Total FAAs | ① | 25.30 | 20.35 | 45.93 | 19.75 | 23.19 | 10.30 | 35.40 | 18.09 | 16.50 | 12.80 | 20.66 | 4.22 |
| ② | 27.34 | 10.00 | 40.57 | 20.54 | 17.50 | 7.29 | 33.40 | 13.42 | 19.76 | 8.79 | 13.92 | 2.65 | |
| ③ | 23.38 | 5.71 | 17.56 | 8.23 | 12.62 | 6.15 | 9.79 | 4.25 | 8.55 | 10.19 | 6.44 | 6.01 | |
| ④ | 19.53 | -- | 18.57 | -- | 21.23 | -- | -- | -- | 14.35 | 7.14 | 24.43 | 6.84 | |
| FAA | Disinfectant | DBPs | Reference |
|---|---|---|---|
| Ala | Chlorination | MCAld, DCAld, TCAld, CF | [5] |
| Thr | Chlorination | TCAce | [30] |
| Leu | Chlorination | CF | [31] |
| Asp | Chlorination | CH, DCAN, TCNM, DcAcAm, CF | [3] [31] |
| Phe | Chlorination | Phenylacetonitrile, phenylacetaldehyde, benzyl chloride, 2-chlorobenzyl cyanide, 2, 6-diphenylpyridine | [32] |
| Glu | Chlorination | CF | [31] |
| Tyr | Chlorination | CH, DCAN, TCAN, DCAA, TCAA, TCNM, CNCl,4-CP | [23] [3,23] [3] |
| Gly | Chlorination | THMs, CF, DCAN, CNCl | [33] [3] |
| Trp | Chlorination | CH, DCAN | [3] |
| His | Chlorination | CF, DCAN, DCAcAm | [33] |
| Arg | Chlorination | CF | [31] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yu, Q.; Zhou, R.; Feng, J.; Zhang, K.; Li, X.; Ma, X.; Dietrich, A.M. Occurrence of Free Amino Acids in the Source Waters of Zhejiang Province, China, and Their Removal and Transformation in Drinking Water Systems. Water 2020, 12, 73. https://doi.org/10.3390/w12010073
Yang Y, Yu Q, Zhou R, Feng J, Zhang K, Li X, Ma X, Dietrich AM. Occurrence of Free Amino Acids in the Source Waters of Zhejiang Province, China, and Their Removal and Transformation in Drinking Water Systems. Water. 2020; 12(1):73. https://doi.org/10.3390/w12010073
Chicago/Turabian StyleYang, Yulong, Qi Yu, Ruonan Zhou, Jiao Feng, Kejia Zhang, Xueyan Li, Xiaoyan Ma, and Andrea M. Dietrich. 2020. "Occurrence of Free Amino Acids in the Source Waters of Zhejiang Province, China, and Their Removal and Transformation in Drinking Water Systems" Water 12, no. 1: 73. https://doi.org/10.3390/w12010073
APA StyleYang, Y., Yu, Q., Zhou, R., Feng, J., Zhang, K., Li, X., Ma, X., & Dietrich, A. M. (2020). Occurrence of Free Amino Acids in the Source Waters of Zhejiang Province, China, and Their Removal and Transformation in Drinking Water Systems. Water, 12(1), 73. https://doi.org/10.3390/w12010073