A Comparison of the Mechanism of TOC and COD Degradation in Rhodamine B Wastewater by a Recycling-Flow Two- and Three-dimensional Electro-Reactor System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
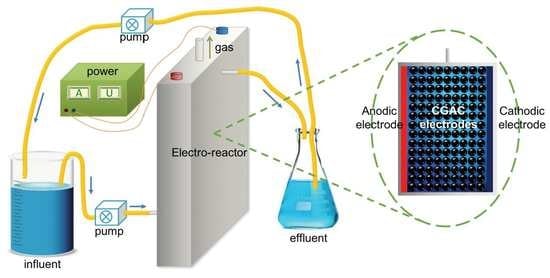
2.2. Experimental Setup and Procedure
2.3. Analytical Methods
3. Result and Discussion
3.1. Electrochemical Properties of TOC and COD Degradation in the Recycling-Flow Elecro-Reactor System
3.2. Mechanism of TOC and COD Degradation with Different Electrolytes in the Recycling-Flow Electro-Reactor System
3.3. Mechanism of TOC and COD Degradation in Different Voltages in the Recycling-Flow Electro-Reactor System
3.4. Mechanism of TOC and COD Degradation at Different HRTs in the Recycling-Flow Electro-Reactor System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Massoud, M.A.; Tarhini, A.; Nasr, J.A. Decentralized approaches to wastewater treatment and management: Applicability in developing countries. J. Environ. Manag. 2009, 90, 652–659. [Google Scholar] [CrossRef]
- Naumczyka, J.H.; Kucharskab, M.A.; Ładyńskab, J.A.; Wojewódkaa, D. Electrochemical oxidation process in application to raw and biologically pre-treated tannery wastewater. Desalin. Water Treat. 2019, 162, 166–175. [Google Scholar] [CrossRef]
- Cui, M.-H.; Gao, J.; Wang, A.-J.; Sangeetha, T. Azo dye wastewater treatment in a bioelectrochemical-aerobic integrated system: Effect of initial azo dye concentration and aerobic sludge concentration. Desalin. Water Treat. 2019, 165, 314–320. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, H.; Dimassimo, R.; Xu, G. Pilot scale study of sequencing batch reactor (sbr) retrofit with integrated fixed film activated sludge (ifas): Nitrogen removal and design consideration. Environ. Sci. Water Res. Technol. 2018, 4, 569–581. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Kim, S.; Lee, J.; Yoon, J. Electrochemical lithium recovery and organic pollutant removal from industrial wastewater of a battery recycling plant. Environ. Sci. Water Res. Technol. 2018, 4, 175–182. [Google Scholar] [CrossRef]
- Soares, P.A.; Batalha, M.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Enhancement of a solar photo-fenton reaction with ferric-organic ligands for the treatment of acrylic-textile dyeing wastewater. J. Environ. Manag. 2015, 152, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, S.; Barzegar, G.; Ahmadi, M.; Soltani, R.D.C.; Takdastan, A.; Saeedi, R.; Abtahi, M. Enhanced coagulation-photocatalytic treatment of acid red 73 dye and real textile wastewater using uva/synthesized mgo nanoparticles. J. Environ. Manag. 2016, 177, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Le Luua, T.; Tiena, T.T.; Duongb, N.B.; Phuongb, N.T.T. Study of the treatment of tannery wastewater after biological pretreatment by using electrochemical oxidation on bdd/ti anode. Desalin. Water Treat. 2019, 137, 194–201. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Wang, C.; Zhang, L.; Qian, Y.; Xue, F.; Wu, Y. The electrochemical oxidation of biologically treated citric acid wastewater in a continuous-flow three-dimensional electrode reactor (ctder). Chem. Eng. J. 2013, 232, 495–502. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Yousefi, Z.; Zafarzadeh, A.; Mohammadpour, R.A.; Zarei, E.; Mengelizadeh, N.; Ghezel, A. Electrochemical removal of acid red 18 dye from synthetic wastewater using a three-dimensional electrochemical reactor. Desalin. Water Treat. 2019, 165, 352–361. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Hou, Y.; Peng, Z.; Wang, L.; Gong, Z.; Zhu, J.; Su, D. Highly efficient pd-fe/ni foam as heterogeneous fenton catalysts for the three-dimensional electrode system. Catal. Commun. 2016, 86, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Qiying, L.; Hongyu, S.; Xibo, L.; Junwu, X.; Fei, X.; Limin, L.; Jun, L.; Shuai, W. Ultrahigh capacitive performance of three-dimensional electrode nanomaterials based on α -mno 2 nanocrystallines induced by doping au through Å-scale channels. Nano Energy 2016, 21, 39–50. [Google Scholar]
- Yu, X.; Hua, T.; Liu, X.; Yan, Z.; Xu, P.; Du, P. Nickel-based thin film on multiwalled carbon nanotubes as an efficient bifunctional electrocatalyst for water splitting. Acs Appl. Mater. Interfaces 2014, 6, 15395–15402. [Google Scholar] [CrossRef]
- Yu, X.; Sun, Z.; Yan, Z.; Xiang, B.; Liu, X.; Du, P. Direct growth of porous crystalline nico2o4 nanowire arrays on a conductive electrode for high-performance electrocatalytic water oxidation. J. Mater. Chem. A 2014, 2, 20823–20831. [Google Scholar] [CrossRef]
- Yu, X.; Xu, P.; Hua, T.; Han, A.; Liu, X.; Wu, H.; Du, P. Multi-walled carbon nanotubes supported porous nickel oxide as noble metal-free electrocatalysts for efficient water oxidation. Int. J. Hydrog. Energy 2014, 39, 10467–10475. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.H.; Lim, T.T. Enhanced electrochemical oxidation of phenol using hydrophobic tio2-nts/sno2-sb-ptfe electrode prepared by pulse electrodeposition. RSC Adv. 2015, 5, 32245–32255. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhu, W.; Xue, F.; Qian, Y.; Wang, C. Enhanced electrochemical oxidation of synthetic dyeing wastewater using sno 2 -sb-doped tio 2 -coated granular activated carbon electrodes with high hydroxyl radical yields. Electrochim. Acta 2016, 220, 276–284. [Google Scholar] [CrossRef]
- Liu, W.; Ai, Z.; Zhang, L. Design of a neutral three-dimensional electro-fenton system with foam nickel as particle electrodes for wastewater treatment. J. Hazard. Mater. 2012, 243, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-y.; Li, N.; Zhao, L. Three-dimensional electrode microbial fuel cell for hydrogen peroxide synthesis coupled to wastewater treatment. J. Power Sources 2014, 254, 316–322. [Google Scholar] [CrossRef]
- Hao, R.; Li, S.; Li, J.; Meng, C. Denitrification of simulated municipal wastewater treatment plant effluent using a three-dimensional biofilm-electrode reactor: Operating performance and bacterial community. Bioresour. Technol. 2013, 143, 178–186. [Google Scholar] [CrossRef]
- Neti, N.R.; Misra, R. Efficient degradation of reactive blue 4 in carbon bed electrochemical reactor. Chem. Eng. J. 2012, 184, 23–32. [Google Scholar] [CrossRef]
- Pang, T.; Wang, Y.; Yang, H.; Wang, T.; Cai, W. Dynamic model of organic pollutant degradation in three dimensional packed bed electrode reactor. Chemosphere 2018, 206, 107–114. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Li, Y.; Xu, T.; Zhu, S. Continuous electrochemical oxidation of methyl orange waste water using a three-dimensional electrode reactor. J. Environ. Sci. 2011, 23, S70–S73. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Shi, Z.; Fang, Y.; Shi, S.; Wang, J.; Wu, C. Advanced treatment of wet-spun acrylic fiber manufacturing wastewater using three-dimensional electrochemical oxidation. J. Environ. Sci. 2016, 50, 21–31. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Sun, Y.; Xu, L.N.; Ni, J.R. Removal of acid orange 7 in simulated wastewater using a three-dimensional electrode reactor: Removal mechanisms and dye degradation pathway. Chemosphere 2010, 78, 46–51. [Google Scholar] [CrossRef]
- Wei, L.; Guo, S.; Yan, G.; Chen, C.; Jiang, X. Electrochemical pretreatment of heavy oil refinery wastewater using a three-dimensional electrode reactor. Electrochim. Acta 2010, 55, 8615–8620. [Google Scholar] [CrossRef]
- Jung, K.-W.; Hwang, M.-J.; Park, D.-S.; Ahn, K.-H. Performance evaluation and optimization of a fluidized three-dimensional electrode reactor combining pre-exposed granular activated carbon as a moving particle electrode for greywater treatment. Sep. Purif. Technol. 2015, 156, 414–423. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Z.; Liu, Y.; Yang, G. Preparation of organosolv lignin-stabilized nano zero-valent iron and its application as granular electrode in the tertiary treatment of pulp and paper wastewater. Chem. Eng. J. 2018, 331, 317–325. [Google Scholar] [CrossRef]
- Can, W.; Yao-Kun, H.; Qing, Z.; Min, J. Treatment of secondary effluent using a three-dimensional electrode system: Cod removal, biotoxicity assessment, and disinfection effects. Chem. Eng. J. 2014, 243, 1–6. [Google Scholar] [CrossRef]
- Li, X.-Y.; Xu, J.; Cheng, J.-P.; Feng, L.; Shi, Y.-F.; Ji, J. Tio2-sio2/gac particles for enhanced electrocatalytic removal of acid orange 7 (ao7) dyeing wastewater in a three-dimensional electrochemical reactor. Sep. Purif. Technol. 2017, 187, 303–310. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Yu, Z.; Liu, Y.; Huang, J.; Qian, L.; Xiong, J. Three-dimensional electro-fenton degradation of rhodamine b with efficient fe-cu/kaolin particle electrodes: Electrodes optimization, kinetics, influencing factors and mechanism. Sep. Purif. Technol. 2019, 210, 60–68. [Google Scholar] [CrossRef]
- Chen, H.; Feng, Y.; Suo, N.; Long, Y.; Li, X.; Shi, Y.; Yu, Y. Preparation of particle electrodes from manganese slag and its degradation performance for salicylic acid in the three-dimensional electrode reactor (tde). Chemosphere 2019, 216, 281–288. [Google Scholar] [CrossRef]
- He, W.; Ma, Q.; Wang, J.; Yu, J.; Bao, W.; Ma, H.; Amrane, A. Preparation of novel kaolin-based particle electrodes for treating methyl orange wastewater. Appl. Clay Sci. 2014, 99, 178–186. [Google Scholar] [CrossRef]
- Zhan, J.; Li, Z.; Yu, G.; Pan, X.; Wang, J.; Zhu, W.; Han, X.; Wang, Y. Enhanced treatment of pharmaceutical wastewater by combining three-dimensional electrochemical process with ozonation to in situ regenerate granular activated carbon particle electrodes. Sep. Purif. Technol. 2019, 208, 12–18. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in electro-fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, M.; Ren, G.; Ma, L. A novel dual gas diffusion electrodes system for efficient hydrogen peroxide generation used in electro-fenton. Chem. Eng. J. 2015, 263, 92–100. [Google Scholar] [CrossRef]
- Wang, C.-T.; Chou, W.-L.; Chung, M.-H.; Kuo, Y.-M. Cod removal from real dyeing wastewater by electro-fenton technology using an activated carbon fiber cathode. Desalination 2010, 253, 129–134. [Google Scholar] [CrossRef]
- Wang, C.T.; Hu, J.L.; Chou, W.L.; Kuo, Y.M. Removal of color from real dyeing wastewater by electro-fenton technology using a three-dimensional graphite cathode. J. Hazard. Mater. 2008, 152, 601–606. [Google Scholar] [CrossRef]
- Lei, H.; Li, H.; Li, Z.; Li, Z.; Chen, K.; Zhang, X.; Wang, H. Electro-fenton degradation of cationic red x-grl using an activated carbon fiber cathode. Process Saf. Environ. Prot. 2010, 88, 431–438. [Google Scholar] [CrossRef]
- Pérez, J.F.; Sabatino, S.; Galia, A.; Rodrigo, M.A.; Llanos, J.; Sáez, C.; Scialdone, O. Effect of air pressure on the electro-fenton process at carbon felt electrodes. Electrochim. Acta 2018, 273, 447–453. [Google Scholar] [CrossRef]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Submerged anaerobic membrane bioreactor for low-strength wastewater treatment: Effect of hrt and srt on treatment performance and membrane fouling. Water Res. 2011, 45, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Yang, W.N.; Ngo, H.H.; Guo, W.S.; Jin, P.K.; Dzakpasu, M.; Yang, S.J.; Wang, Q.; Wang, X.C.; Ao, D. Current status of urban wastewater treatment plants in china. Environ. Int. 2016, 92, 11–22. [Google Scholar] [CrossRef]












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, J.; Shi, H.; Xu, Y.; Wang, Q. A Comparison of the Mechanism of TOC and COD Degradation in Rhodamine B Wastewater by a Recycling-Flow Two- and Three-dimensional Electro-Reactor System. Water 2020, 12, 1853. https://doi.org/10.3390/w12071853
Ni J, Shi H, Xu Y, Wang Q. A Comparison of the Mechanism of TOC and COD Degradation in Rhodamine B Wastewater by a Recycling-Flow Two- and Three-dimensional Electro-Reactor System. Water. 2020; 12(7):1853. https://doi.org/10.3390/w12071853
Chicago/Turabian StyleNi, Jin, Huimin Shi, Yuansheng Xu, and Qunhui Wang. 2020. "A Comparison of the Mechanism of TOC and COD Degradation in Rhodamine B Wastewater by a Recycling-Flow Two- and Three-dimensional Electro-Reactor System" Water 12, no. 7: 1853. https://doi.org/10.3390/w12071853
APA StyleNi, J., Shi, H., Xu, Y., & Wang, Q. (2020). A Comparison of the Mechanism of TOC and COD Degradation in Rhodamine B Wastewater by a Recycling-Flow Two- and Three-dimensional Electro-Reactor System. Water, 12(7), 1853. https://doi.org/10.3390/w12071853