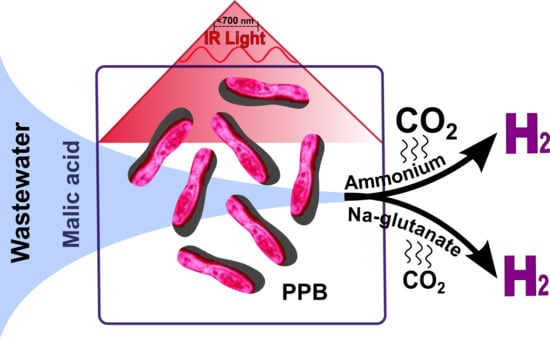
Optimization of H2 Production through Minimization of CO2 Emissions by Mixed Cultures of Purple Phototrophic Bacteria in Aqueous Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Compounds
2.2. Enrichment of Purple Phototrophic Bacteria (PPB)
2.3. Batch Assays of PPB
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. PPB Culture Enrichment
3.2. Effect of Nitrogen Sources on H2 and CO2 Production
3.3. Effect of Carbon Sources on H2 and CO2 Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Batstone, D.J.; Hülsen, T.; Mehta, C.M.; Keller, J. Platforms for energy and nutrient recovery from domestic wastewater: A review. Chemosphere 2015, 140, 2–11. [Google Scholar] [CrossRef]
- Garcia, D.J.; You, F. The water-energy-food nexus and process systems engineering: A new focus. Comput. Chem. Eng. 2016, 91, 49–67. [Google Scholar] [CrossRef]
- Hülsen, T.; Batstone, D.J.; Keller, J. Phototrophic bacteria for nutrient recovery from domestic wastewater. Water Res. 2014, 50, 18–26. [Google Scholar] [CrossRef]
- Zimmo, O.; van der Steen, P.; Gijzen, H. Nitrogen mass balance across pilot-scale algae and duckweed-based wastewater stabilisation ponds. Water Res. 2004, 38, 913–920. [Google Scholar] [CrossRef]
- Lavrinovičs, A.; Juhna, T. Review on Challenges and Limitations for Algae-Based Wastewater Treatment. Constr. Sci. 2017, 20, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Hülsen, T.; Barry, E.M.; Lu, Y.; Puyol, D.; Batstone, D.J. Low temperature treatment of domestic wastewater by purple phototrophic bacteria: Performance, activity, and community. Water Res. 2016, 100, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Yang, H.; Huang, Z.; Liu, S.-J. Rhodocista pekingensis sp. nov., a cyst-forming phototrophic bacterium from a municipal wastewater treatment plant. Int. J. Syst. Evol. Microbiol. 2003, 53, 1111–1114. [Google Scholar] [CrossRef]
- Basak, N.; Das, D. The Prospect of Purple Non-Sulfur (PNS) Photosynthetic Bacteria for Hydrogen Production: The Present State of the Art. World J. Microbiol. Biotechnol. 2006, 23, 31–42. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, K.-M.; Yin, C.-R.; Lee, K.-Y.; Im, W.-T.; Lim, J.H.; Lee, S.-T. Odorous swine wastewater treatment by purple non-sulfur bacteria, Rhodopseudomonas palustris, isolated from eutrophicated ponds. Biotechnol. Lett. 2004, 26, 819–822. [Google Scholar] [CrossRef]
- Koku, H. Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides. Int. J. Hydrog. Energy 2002, 27, 1315–1329. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Harwood, C.S. Carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 11669–11675. [Google Scholar] [CrossRef] [Green Version]
- Mckinlay, J.B.; Harwood, C.S. Calvin Cycle Flux, Pathway Constraints, and Substrate Oxidation State Bacteria. mBio 2011, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Puyol, D.; Barry, E.; Hülsen, T.; Batstone, D.J. A mechanistic model for anaerobic phototrophs in domestic wastewater applications: Photo-anaerobic model (PAnM). Water Res. 2017, 116, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Rey, F.E.; Heiniger, E.; Harwood, C.S. Redirection of Metabolism for Biological Hydrogen Production. Appl. Environ. Microbiol. 2007, 73, 1665–1671. [Google Scholar] [CrossRef] [Green Version]
- Vasiliadou, I.; Berná, A.; Manchon, C.; Melero, J.A.; Martinez, F.; Esteve-Nuñez, A.; Puyol, D. Biological and Bioelectrochemical Systems for Hydrogen Production and Carbon Fixation Using Purple Phototrophic Bacteria. Front. Energy Res. 2018, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, L.S.; Kontur, W.S.; Sanders, A.P.; Sohmen, U.; Donohue, T.J.; Noguera, D.R. Electron Partitioning During Light- and Nutrient-Powered Hydrogen Production by Rhodobacter sphaeroides. BioEnergy Res. 2010, 3, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y. Characteristics of a new photosynthetic bacterial strain for hydrogen production and its application in wastewater treatment. Int. J. Hydrog. Energy 2008, 33, 963–973. [Google Scholar] [CrossRef]
- Sasikala, C.H.; Ramana, C.H.V.; Rao, P.R. Regulation of simultaneous hydrogen photoproduction during growth by pH and glutamate in Rhodobacter sphaeroides O.U. 001. Int. J. Hydrog. Energy 1995, 20, 123–126. [Google Scholar] [CrossRef]
- Ventura, R.L.G.; Ventura, J.R.S.; Oh, Y.S. Photoheterotrophic hydrogen production of rhodobacter sphaeroides KCTC 1434 under alternating ar and N2 headspace gas. Philipp. J. Sci. 2019, 148, 63–72. [Google Scholar]
- Fang, H.; Liu, H.; Zhang, T. Phototrophic hydrogen production from acetate and butyrate in wastewater. Int. J. Hydrog. Energy 2005, 30, 785–793. [Google Scholar] [CrossRef]
- Lee, H.-S.; Vermaas, W.F.; Rittmann, B.E. Biological hydrogen production: Prospects and challenges. Trends Biotechnol. 2010, 28, 262–271. [Google Scholar] [CrossRef]
- Craven, J.; Sultan, M.A.; Sarma, R.; Wilson, S.; Meeks, N.; Kim, D.Y.; Hastings, J.T.; Bhattacharyya, D. Rhodopseudomonas palustris-based conversion of organic acids to hydrogen using plasmonic nanoparticles and near-infrared light. RSC Adv. 2019, 9, 41218–41227. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.Y.; Hay, J.X.W.; Kong, L.B.; Juan, J.C.; Jahim, J.M. Recent advances in reuse of waste material as substrate to produce biohydrogen by purple non-sulfur (PNS) bacteria. Renew. Sustain. Energy Rev. 2012, 16, 3117–3122. [Google Scholar] [CrossRef]
- Chiemchaisri, C.; Jaitrong, L.; Honda, R.; Fukushi, K.; Yamamoto, K. Photosynthetic bacteria pond system with infra-red transmitting filter for the treatment and recovery of organic carbon from industrial wastewater. Water Sci. Technol. 2007, 56, 109–116. [Google Scholar] [CrossRef]
- Lee, M.G.; Kobayashi, M. Deodorization of swine sewage by addition of a phototrophic bacterium, Rhodopseudomonas capsulata. Soil Sci. Plant Nutr. 1992, 38, 767–770. [Google Scholar] [CrossRef] [Green Version]
- Lo, Y.-C.; Chen, C.-Y.; Lee, C.-M.; Chang, J.-S. Photo fermentative hydrogen production using dominant components (acetate, lactate, and butyrate) in dark fermentation effluents. Int. J. Hydrog. Energy 2011, 36, 14059–14068. [Google Scholar] [CrossRef]
- Tawfik, A.; El-Bery, H.; Singh, G.; Bux, F. Use of mixed culture bacteria for photofermentive hydrogen of dark fermentation effluent. Bioresour. Technol. 2014, 168, 119–126. [Google Scholar] [CrossRef]
- Kantachote, D.; Torpee, S.; Umsakul, K. The potential use of anoxygenic phototrophic bacteria for treating latex rubber sheet wastewater. Electron. J. Biotechnol. 2005, 8, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Suzuki, T.; Tsygankov, A.; Asada, Y.; Miyake, J. Hydrogen production from tofu wastewater by Rhodobacter sphaeroides immobilized in agar gels. Int. J. Hydrog. Energy 1999, 24, 305–310. [Google Scholar] [CrossRef]
- Yetis, M. Photoproduction of hydrogen from sugar refinery wastewater by Rhodobacter sphaeroides O.U. 001. Int. J. Hydrog. Energy 2000, 25, 1035–1041. [Google Scholar] [CrossRef]
- Serna, R.L.; Garcia, D.; Bolado, S.; Jiménez, J.J.; Lai, F.Y.; Golovko, O.; Gago-Ferrero, P.; Ahrens, L.; Wiberg, K.; Munoz, R. Photobioreactors based on microalgae-bacteria and purple phototrophic bacteria consortia: A promising technology to reduce the load of veterinary drugs from piggery wastewater. Sci. Total. Environ. 2019, 692, 259–266. [Google Scholar] [CrossRef]
- Hülsen, T.; Hsieh, K.; Lu, Y.; Tait, S.; Batstone, D.J. Simultaneous treatment and single cell protein production from agri-industrial wastewaters using purple phototrophic bacteria or microalgae—A comparison. Biores. Technol. 2018, 254, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Hülsen, T.; Hsieh, K.; Tait, S.; Barry, E.M.; Puyol, D.; Batstone, D.J. White and infrared light continuous photobioreactors for resource recovery from poultry processing wastewater—A comparison. Water Res. 2018, 144, 665–676. [Google Scholar] [CrossRef]
- Hülsen, T.; Hsieh, K.; Batstone, D.J. Saline wastewater treatment with purple phototrophic bacteria. Water Res. 2019, 160, 259–267. [Google Scholar] [CrossRef]
- Dalaei, P.; Bahreini, G.; Nakhla, G.; Santoro, D.; Batstone, D.; Hülsen, T. Municipal wastewater treatment by purple phototropic bacteria at low infrared irradiances using a photo-anaerobic membrane bioreactor. Water Res. 2020, 173, 115535. [Google Scholar] [CrossRef]
- Hu, J.; Yang, H.; Wang, X.; Cao, W.; Guo, L. Strong pH dependence of hydrogen production from glucose by Rhodobacter sphaeroides. Int. J. Hydrog. Energy 2020, 45, 9451–9458. [Google Scholar] [CrossRef]
- Ormerod, J.G.; Ormerod, K.S.; Gest, H. Light-Dependent Utilization of Organic Compounds Photoproduction of Molecular Hydrogen by Photosynthetic Bacteria; Relationships with Nitrogen Metabolism’ of organic com- grown with N, or certain amino acids pro- duce large quantities of molecular hydr. Arch. Biochem. Biophys. 1961, 94, 449–463. [Google Scholar] [PubMed]
- Clemens, P.; Walter, C. Microalgal Biotechnology: Potential and Production; Walter de Gruyter: Berlin, Germany, 2012; ISBN 978-3-11-022501-3. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- Heras, I.D.L.; Molina, R.; Segura, Y.; Hülsen, T.; Molina, M.; Gonzalez-Benítez, N.; Melero, J.; de la Rubia, M.; Martínez, F.; Puyol, D. Contamination of N-poor wastewater with emerging pollutants does not affect the performance of purple phototrophic bacteria and the subsequent resource recovery potential. J. Hazard. Mater. 2020, 385, 121617. [Google Scholar] [CrossRef]
- Melnicki, M.; Bianchi, L.; de Philippis, R.; Melis, A. Hydrogen production during stationary phase in purple photosynthetic bacteria. Int. J. Hydrog. Energy 2008, 33, 6525–6534. [Google Scholar] [CrossRef]
- Niedzwiedzki, D.M.; Dilbeck, P.L.; Tang, Q.; Martin, E.C.; Bocian, D.F.; Hunter, C.N.; Holten, D. New insights into the photochemistry of carotenoid spheroidenone in light-harvesting complex 2 from the purple bacterium Rhodobacter sphaeroides. Photosynth. Res. 2016, 131, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Kim, D.-H.; Cha, J. Culture conditions affecting H2 production by phototrophic bacterium Rhodobacter sphaeroides KD131. Int. J. Hydrog. Energy 2012, 37, 14055–14061. [Google Scholar] [CrossRef]
- Tichi, M.A.; Tabita, F.R. Maintenance and control of redox poise in Rhodobacter capsulatus strains deficient in the Calvin-Benson-Bassham pathway. Arch. Microbiol. 2000, 174, 322–333. [Google Scholar] [CrossRef]
- Wang, X.; Falcone, D.L.; Tabita, F.R. Reductive pentose phosphate-independent CO2 fixation in Rhodobacter sphaeroides and evidence that ribulose bisphosphate carboxylase/oxygenase activity serves to maintain the redox balance of the cell. J. Bacteriol. 1993, 175, 3372–3379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasikala, K.; Ramana, C.; Rao, P.R.; Kovács, K. Anoxygenic Phototrophic Bacteria: Physiology and Advances in Hydrogen Production Technology. Nat. Eng. Resist. Plant Vir. Part II 1993, 38, 211–295. [Google Scholar] [CrossRef]
- Hillmer, P.; Gest, H. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: Production and utilization of H2 by resting cells. J. Bacteriol. 1977, 129, 732–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eroglu, N.; Aslan, K.; Gündüz, U.; Yücel, M.; Turker, L. Substrate consumption rates for hydrogen production by Rhodobacter sphaeroidesin a column photobioreactor. Biotransform. Microb. Degrada. Health-Risk Compd. 1999, 35, 103–113. [Google Scholar] [CrossRef]
- Eroglu, I. Hydrogen production by Rhodobacter sphaeroides O.U.001 in a flat plate solar bioreactor. Int. J. Hydrog. Energy 2008, 33, 531–541. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Miyake, J.; Kawamura, S. Screening photosynthesis bacteria for hydrogen production from organic acids. J. Ferment. Technol. 1986, 64, 245–249. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Rocha, J.; Tramper, J.; Wijffels, R.H. Acetate as a carbon source for hydrogen production by photosynthetic bacteria. J. Biotechnol. 2001, 85, 25–33. [Google Scholar] [CrossRef]








| RUN Number | Carbon Source | Nitrogen Source | Nitrogen (mgN/L) | COD-Substrate to N Ratio a COD:N | Measured COD to N Ratio b COD:N |
|---|---|---|---|---|---|
| R1 | Malic acid | NH4Cl | 75 | 100:3.8 | 100:3 |
| R2 | 150 | 100:7.5 | 100:6.8 | ||
| R3 | 300 | 100:15 | 100:12.1 | ||
| R4 | 600 | 100:30 | 100:33.8 | ||
| R5 | Acetic acid | NH4Cl | 75 | 100:3.8 | 100:2.9 |
| R6 | 150 | 100:7.5 | 100:8.6 | ||
| R7 | 300 | 100:15 | 100:15.6 | ||
| R8 | 600 | 100:30 | 100:44 | ||
| R9 | Malic acid | Na-glutamate | 75 | 100:3.8 | 100:2.3 |
| R10 | 150 | 100:7.5 | 100:4.6 | ||
| R11 | 300 | 100:15 | 100:5.5 | ||
| R12 | Butyric acid | Na-glutamate | 300 | 100:15 | 100:6.9 |
| R13 | Acetic acid | Na-glutamate | 300 | 100:15 | 100:6.7 |
| R14 | Malic acid | Ν2 gas | 650 c | 100:33 | 100:36 |
| RUN Number | Carbon Source | Nitrogen Source | Nitrogen (mgN/L) | COD-Substrate to N Ratio a COD:N | Measured COD to N Ratio b COD:N | RPPB c (mgVSS/Lh) | Racid d (mM acid/h) | H2 e (mLH2/L) | H2_rate f (mLH2/Lh) | YH2 g (LH2/g_Acid) | CO2 h (mLCO2/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | Malic acid | NH4Cl | 75 | 100:3.8 | 100:3 | 3.7 ± 0.8 | 0.32 ± 0.11 | 381.6 ± 13.2 | 2.5 ± 0.8 | 0.11 ± 0.01 | 51.2 ± 1.6 |
| R2 | 150 | 100:7.5 | 100:6.8 | 3.7 ± 1.7 | 0.28 ± 0.10 | 1.4 ± 2.1 | 0 ± 0 | 0 ± 0 | 5.6 ± 3.0 | ||
| R3 | 300 | 100:15 | 100:12.1 | 4.2 ± 2.3 | 0.26 ± 0.11 | 1.8 ± 2.9 | 0 ± 0 | 0 ± 0 | 11.0 ± 1.7 | ||
| R4 | 600 | 100:30 | 100:33.8 | 1.69 | 0.31 | 0 | 0 | 0 | 15.1 | ||
| R5 | Acetic acid | NH4Cl | 75 | 100:3.8 | 100:2.9 | 3.62 | 0.11 | 0 | 0.01 | 0.002 | 0 |
| R6 | 150 | 100:7.5 | 100:8.6 | 2.93 | 0.07 | 2.4 | 0.02 | 0.004 | 0 | ||
| R7 | 300 | 100:15 | 100:15.6 | 3.95 | 0.12 | 2.9 | 0.02 | 0.002 | 0 | ||
| R8 | 600 | 100:30 | 100:44 | 4.30 | 0.09 | 2.8 | 0 | 0 | 0.2 | ||
| R9 | Malic acid | Na-glutamate | 75 | 100:3.8 | 100:2.3 | 3.8 ± 0.5 | 0.31 ± 0.08 | 264.3 ± 96.4 | 1.7 ± 0.8 | 0.08 ± 0.03 | 66.4 ± 6.1 |
| R10 | 150 | 100:7.5 | 100:4.6 | 4.6 ± 2.9 | 0.23 ± 0.12 | 305.9 ± 64.2 | 2.1 ± 0.9 | 0.12 ± 0.05 | 38.2 ± 21.4 | ||
| R11 | 300 | 100:15 | 100:5.5 | 6.9 ± 2.4 | 0.30 ± 0.05 | 370.0 ± 27.4 | 2.3 ± 0.2 | 0.11 ± 0.01 | 13.4 ± 13.6 | ||
| R12 | Butyric acid | Na-glutamate | 300 | 100:15 | 100:6.9 | 2.7 ± 0.9 | 0.04 ± 0.03 | 145.2 ± 31.6 | 0.86 ± 0.19 | 0.40 ± 0.28 | 1.1 ± 1.3 |
| R13 | Acetic acid | Na-glutamate | 300 | 100:15 | 100:6.7 | 3.3 ± 0.9 | 0.10 ± 0.08 | 94.6 ± 7.5 | 0.56 ± 0.04 | 0.16 ± 0.15 | 0.10 ± 0.12 |
| R14 | Malic acid | Ν2 gas | 650 | 100:33 | 100:36 | 3.3 ± 2.1 | 0.01 ± 0 | 1.7 ± 0 | 0 ± 0 | 0 ± 0 | 1.6 ± 1.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliadou, I.A.; Melero, J.A.; Molina, R.; Puyol, D.; Martinez, F. Optimization of H2 Production through Minimization of CO2 Emissions by Mixed Cultures of Purple Phototrophic Bacteria in Aqueous Samples. Water 2020, 12, 2015. https://doi.org/10.3390/w12072015
Vasiliadou IA, Melero JA, Molina R, Puyol D, Martinez F. Optimization of H2 Production through Minimization of CO2 Emissions by Mixed Cultures of Purple Phototrophic Bacteria in Aqueous Samples. Water. 2020; 12(7):2015. https://doi.org/10.3390/w12072015
Chicago/Turabian StyleVasiliadou, I.A., J.A. Melero, R. Molina, D. Puyol, and F. Martinez. 2020. "Optimization of H2 Production through Minimization of CO2 Emissions by Mixed Cultures of Purple Phototrophic Bacteria in Aqueous Samples" Water 12, no. 7: 2015. https://doi.org/10.3390/w12072015
APA StyleVasiliadou, I. A., Melero, J. A., Molina, R., Puyol, D., & Martinez, F. (2020). Optimization of H2 Production through Minimization of CO2 Emissions by Mixed Cultures of Purple Phototrophic Bacteria in Aqueous Samples. Water, 12(7), 2015. https://doi.org/10.3390/w12072015