Sorption Characteristics and Removal Efficiency of Organic Micropollutants in Drinking Water Using Granular Activated Carbon (GAC) in Pilot-Scale and Full-Scale Tests
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Full-Scale GAC Treatment at a DWTP
2.3. Pilot-Scale GAC Column Tests at a DWTP
2.4. Organic Matter Quantification and Characterization
2.5. Methylene Blue Kinetic Experiments
2.6. OMP Kinetic and Isotherm Experiments
2.7. Sample Preparation and OMP Analysis
2.8. Calculation of Removal Efficiency
3. Results and Discussion
3.1. Kinetic and Isotherm Experiments for OMPs
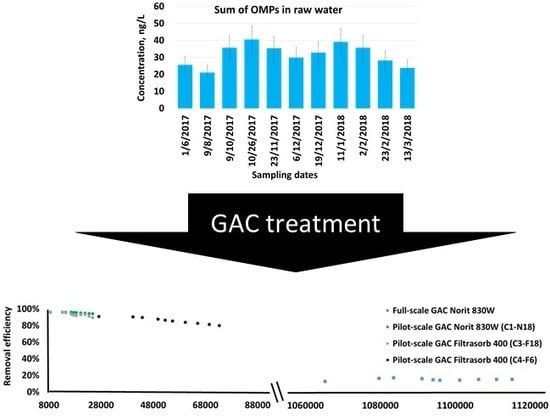
3.2. Removal of OMPs Using GAC in Pilot-Scale Experiments
3.3. Removal of OMPs Using GAC in Full-Scale
3.4. Early Warning Indicators of GAC Sorption Capacity
3.4.1. DOC, UV Absorbance and Fluorescence
3.4.2. Methylene Blue
3.4.3. Online SPE of OMPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- König, M.; Escher, B.I.; Neale, P.A.; Krauss, M.; Hilscherova, K.; Novak, J.; Teodorovic, I.; Schulze, T.; Seidensticker, S.; Kamal Hashmi, M.A.; et al. Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis. Environ. Pollut. 2017, 220, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Rehrl, A.L.; Golovko, O.; Ahrens, L.; Köhler, S. Spatial and seasonal trends of organic micropollutants in Sweden’s most important drinking water reservoir. Chemosphere 2020, 249, 126168. [Google Scholar] [CrossRef] [PubMed]
- Sörengård, M.; Campos-Pereira, H.; Ullberg, M.; Lai, F.Y.; Golovko, O.; Ahrens, L. Mass loads, source apportionment, and risk estimation of organic micropollutants from hospital and municipal wastewater in recipient catchments. Chemosphere 2019, 234, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.M.; Reinert, A.M.; Knappe, D.R.U.; Ferrer, I.; Summers, R.S. Full- and pilot-scale GAC adsorption of organic micropollutants. Water Res. 2015, 68, 238–248. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Stützer, C.; Jekel, M. Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater—Aligning breakthrough curves and capacities. Water Res. 2016, 92, 180–187. [Google Scholar] [CrossRef]
- Zoppini, A.; Ademollo, N.; Amalfitano, S.; Casella, P.; Patrolecco, L.; Polesello, S. Organic priority substances and microbial processes in river sediments subject to contrasting hydrological conditions. Sci. Total Environ. 2014, 484, 74–83. [Google Scholar] [CrossRef]
- Benstoem, F.; Nahrstedt, A.; Boehler, M.; Knopp, G.; Montag, D.; Siegrist, H.; Pinnekamp, J. Performance of granular activated carbon to remove micropollutants from municipal wastewater—A met a-analysis of pilot- and large-scale studies. Chemosphere 2017, 185, 105–118. [Google Scholar] [CrossRef]
- Delgado, L.F.; Charles, P.; Glucina, K.; Morlay, C. The removal of endocrine disrupting compounds, pharmaceutically activated compounds and cyanobacterial toxins during drinking water preparation using activated carbon—A review. Sci. Total Environ. 2012, 435, 509–525. [Google Scholar] [CrossRef]
- Hallé, C.; Huck, P.M.; Peldszus, S. Emerging Contaminant Removal by Biofiltration: Temperature, Concentration, and EBCT Impacts. J. Am. Water Work. Assoc. 2015, 107, E364–E379. [Google Scholar]
- Benstoem, F.; Pinnekamp, J. Characteristic numbers of granular activated carbon for the elimination of micropollutants from effluents of municipal wastewater treatment plants. Water Sci. Technol. 2017, 76, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Deshayes, S.; Zedek, S.; Cren-Olivé, C.; Cartiser, N.; Eudes, V.; Bressy, A.; Caupos, E.; et al. Study of a large scale powdered activated carbon pilot: Removals of a wide range of emerging and priority micropollutants from wastewater treatment plant effluents. Water Res. 2015, 72, 315–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, S.J.; Lavonen, E.; Keucken, A.; Schmitt-Kopplin, P.; Spanjer, T.; Persson, K. Upgrading coagulation with hollow-fibre nanofiltration for improved organic matter removal during surface water treatment. Water Res. 2016, 89, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Lavonen, E.E.; Kothawala, D.N.; Tranvik, L.J.; Gonsior, M.; Schmitt-Kopplin, P.; Köhler, S.J. Tracking changes in the optical properties and molecular composition of dissolved organic matter during drinking water production. Water Res. 2015, 85, 286–294. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Khan, E.; Chen, H.; Nguyen, V.T.; Li, Y.; Goh, S.; Nguyen, Q.B.; Saeidi, N.; Gin, K.Y.H. Emerging contaminants in wastewater, stormwater runoff, and surface water: Application as chemical markers for diffuse sources. Sci. Total Environ. 2019, 676, 252–267. [Google Scholar] [CrossRef]
- Lindberg, R.H.; Östman, M.; Olofsson, U.; Grabic, R.; Fick, J. Occurrence and behaviour of 105 active pharmaceutical ingredients in sewage waters of a municipal sewer collection system. Water Res. 2014, 58, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Removal and seasonal variability of selected analgesics/anti-inflammatory, anti-hypertensive/cardiovascular pharmaceuticals and UV filters in wastewater treatment plant. Environ. Sci. Pollut. Res. 2014, 21, 7578–7585. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Gros, M.; Ahrens, L.; Wiberg, K. Impact of on-site, small and large scale wastewater treatment facilities on levels and fate of pharmaceuticals, personal care products, artificial sweeteners, pesticides, and perfluoroalkyl substances in recipient waters. Sci. Total Environ. 2017, 601, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Tröger, R.; Klöckner, P.; Ahrens, L.; Wiberg, K. Micropollutants in drinking water from source to tap—Method development and application of a multiresidue screening method. Sci. Total Environ. 2018, 627, 1404–1432. [Google Scholar] [CrossRef] [PubMed]
- Lavonen, E.E.; Gonsior, M.; Tranvik, L.J.; Schmitt-Kopplin, P.; Köhler, S.J. Selective Chlorination of Natural Organic Matter: Identification of Previously Unknown Disinfection Byproducts. Environ. Sci. Technol. 2013, 47, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Choi, D.J.; Kim, S.K.; Her, N.; Zoh, K.D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211, 310–317. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- Ort, C.; Lawrence, M.G.; Rieckermann, J.; Joss, A. Sampling for Pharmaceuticals and Personal Care Products (PPCPs) and Illicit Drugs in Wastewater Systems: Are Your Conclusions Valid? A Critical Review. Environ. Sci. Technol. 2010, 44, 6024–6035. [Google Scholar] [CrossRef] [PubMed]
- Young, R.B.; Chefetz, B.; Liu, A.; Desyaterik, Y.; Borch, T. Direct photodegradation of lamotrigine (an antiepileptic) in simulated sunlight – pH influenced rates and products. Environ. Sci. Process. Impacts 2014, 16, 848–857. [Google Scholar] [CrossRef]
- Corwin, C.J.; Summers, R.S. Controlling trace organic contaminants with GAC adsorption. J. Am. Water Work. Assoc. 2012, 104, 43–44. [Google Scholar] [CrossRef]
- Newcombe, G. Charge vs. porosity—Some influences on the adsorption of natural organic matter (NOM) by activated carbon. Water Sci. Technol. 1999, 40, 191–198. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Mead, R.N.; Barefoot, S.; Helms, J.R.; Morgan, J.B.; Kieber, R.J. Photodegradation of the antihistamine cetirizine in natural waters. Environ. Toxicol. Chem. 2014, 33, 2240–2245. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Müller, J.; Sperlich, A.; Ruhl, A.S.; Meinel, F.; Altmann, J.; Jekel, M. Rapid small-scale column testing of granular activated carbon for organic micro-pollutant removal in treated domestic wastewater. Water Sci. Technol. 2014, 70, 1271–1278. [Google Scholar] [CrossRef]
- Anumol, T.; Sgroi, M.; Park, M.; Roccaro, P.; Snyder, S.A. Predicting trace organic compound breakthrough in granular activated carbon using fluorescence and UV absorbance as surrogates. Water Res. 2015, 76, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, O.; Lefevre, B.; Fernandez, M.; Bernat, X.; Paraira, M.; Pons, M. Fractionation and removal of dissolved organic carbon in a full-scale granular activated carbon filter used for drinking water production. Water Res. 2013, 47, 2821–2829. [Google Scholar] [CrossRef] [PubMed]






| Compound | Molecular Formula | Molar Mass (g mol−1) | pKa | Log KOW | Norit 830W | Filtrasorb 400 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kf (ng g−1) | n | R2 | T1/2, min | Kf (ng g−1) | n | R2 | T1/2, min | |||||
| Carbamazepine | C15H12N2O | 236 | 4.2 | 2.3 | 4.3 | 0.95 | 0.997 | 6.7 | 9.3 | 0.97 | 0.924 | 5.7 |
| Cetirizine | C21H25ClN2O3 | 388.9 | 2.7 | 1.7 | 16 | 1.3 | 0.985 | 7.2 | 22 | 1.2 | 0.959 | 5.8 |
| DEET | C12H17NO | 191.1 | 0.67 | 2.3 | 3.7 | 1.2 | 0.989 | 8.9 | 4.6 | 1.2 | 0.979 | 6.5 |
| Fexofenadine | C32H39NO4 | 501.7 | 9.0 | 2.8 | 9.3 | 0.97 | 0.978 | 6.2 | 26 | 0.51 | 0.807 | 6.6 |
| Fluconazole | C13H12F2N6O | 306.1 | 2.0 | 0.25 | 5.2 | 1.1 | 0.987 | 7.0 | 11 | 1.2 | 0.972 | 5.0 |
| Lamotrigine | C9H7Cl2N5 | 255.0 | 5.7 | 0.99 | 11 | 0.78 | 0.976 | 7.4 | 10 | 0.82 | 0.979 | 6.3 |
| Oxazepam | C15H11ClN2O2 | 286.0 | 1.7 | 3.3 | 6 | 1.1 | 0.998 | 6.9 | 11 | 1.1 | 0.977 | 5.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golovko, O.; de Brito Anton, L.; Cascone, C.; Ahrens, L.; Lavonen, E.; Köhler, S.J. Sorption Characteristics and Removal Efficiency of Organic Micropollutants in Drinking Water Using Granular Activated Carbon (GAC) in Pilot-Scale and Full-Scale Tests. Water 2020, 12, 2053. https://doi.org/10.3390/w12072053
Golovko O, de Brito Anton L, Cascone C, Ahrens L, Lavonen E, Köhler SJ. Sorption Characteristics and Removal Efficiency of Organic Micropollutants in Drinking Water Using Granular Activated Carbon (GAC) in Pilot-Scale and Full-Scale Tests. Water. 2020; 12(7):2053. https://doi.org/10.3390/w12072053
Chicago/Turabian StyleGolovko, Oksana, Luana de Brito Anton, Claudia Cascone, Lutz Ahrens, Elin Lavonen, and Stephan J. Köhler. 2020. "Sorption Characteristics and Removal Efficiency of Organic Micropollutants in Drinking Water Using Granular Activated Carbon (GAC) in Pilot-Scale and Full-Scale Tests" Water 12, no. 7: 2053. https://doi.org/10.3390/w12072053
APA StyleGolovko, O., de Brito Anton, L., Cascone, C., Ahrens, L., Lavonen, E., & Köhler, S. J. (2020). Sorption Characteristics and Removal Efficiency of Organic Micropollutants in Drinking Water Using Granular Activated Carbon (GAC) in Pilot-Scale and Full-Scale Tests. Water, 12(7), 2053. https://doi.org/10.3390/w12072053