Comparing the Effects of Types of Electrode on the Removal of Multiple Pharmaceuticals from Water by Electrochemical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical (EC) Experiments and Analyses
3. Results and Discussion
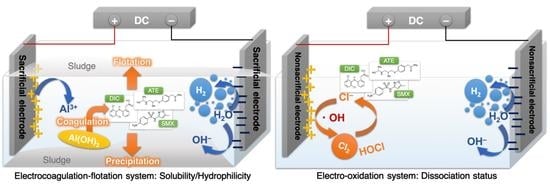
3.1. Removal of ATE, DIC, and SMX with Different Electrode Systems
3.2. Removal Efficiencies of Multiple Pharmaceuticals
3.3. Influences of Multiple Pharmaceuticals on Hospital Wastewater Matrixes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATE | Atenolol |
| DAD | Diode Array Detector |
| DC | Direct Current |
| DIC | Diclofenac |
| EC | Electrochemical |
| HOCl | Hypochlorous Acid |
| HPLC | High-Performance Liquid Chromatography |
| I | Current |
| KH2PO4 | Monopotassium Phosphate |
| NaCl | Sodium Chloride |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| •OH | Hydroxyl Radicals |
| [Phar]0 | Initial Concentration of Pharmaceutical |
| [Phar]s | Initial Concentration of Spiked Pharmaceuticals |
| QA/QC | Quality Assurance/Quality Control |
| SMX | Sulfamethoxazole |
References
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Crouse, B.A.; Ghoshdastidar, A.J.; Tong, A.Z. The presence of acidic and neutral drugs in treated sewage effluents and receiving waters in the Cornwallis and Annapolis River watersheds and the Mill CoveSewage Treatment Plant in Nova Scotia, Canada. Environ. Res. 2012, 112, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Zhang, W.; Xiong, W.; Ye, Q.; Hou, X.; Wang, C.; Wang, P. Life cycle assessment of advanced wastewater treatment processes: Involving 126 pharmaceuticals and personal care products in life cycle inventory. J. Environ. Manag. 2019, 238, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Mestre, A.S.; Pires, R.A.; Aroso, I.; Fernandes, E.M.; Pinto, M.L.; Reis, R.L.; Andrade, M.A.; Pires, J.; Silva, S.P.; Carvalho, A.P. Activated carbons prepared from industrial pre-treated cork: Sustainable adsorbents for pharmaceutical compounds removal. Chem. Eng. J. 2014, 253, 408–417. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Mohamed Saheed, M.S.; Show, P.-L.; Chang, J.-S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Khan, A.H.; Khan, N.A.; Ahmed, S.; Dhingra, A.; Singh, C.P.; Khan, S.U.; Mohammadi, A.A.; Changani, F.; Yousefi, M.; Alam, S.; et al. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J. Clean. Prod. 2020, 269, 122411. [Google Scholar] [CrossRef]
- Petrovic, M.; Hernando, M.D.; Diaz-Cruz, M.S.; Barcelo, D. Liquid chromatography-tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: A review. J. Chromatogr. A 2005, 1067, 1–14. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Berninger, J.P.; Brooks, B.W. Leveraging mammalian pharmaceutical toxicology and pharmacology data to predict chronic fish responses to pharmaceuticals. Toxicol. Lett. 2010, 193, 69–78. [Google Scholar] [CrossRef]
- Camacho-Muñoz, D.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence, temporal evolution and risk assessment of pharmaceutically active compounds in Donana Park (Spain). J. Hazard. Mater. 2010, 183, 602–608. [Google Scholar] [CrossRef]
- Xu, M.; Huang, H.; Li, N.; Li, F.; Wang, D.; Luo, Q. Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotox. Environ. Safe 2019, 175, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Essadki, A.H.; Bennajah, M.; Gourich, B.; Vial, C.; Azzi, M.; Delmas, H. Electrocoagulation/electroflotation in an external-loop airlift reactor—Application to the decolorization of textile dye wastewater: A case study. Chem. Eng. Process. Process Intensif. 2008, 47, 1211–1223. [Google Scholar] [CrossRef]
- Boroski, M.; Rodrigues, A.C.; Garcia, J.C.; Gerola, A.P.; Nozaki, J.; Hioka, N. The effect of operational parameters on electrocoagulation–flotation process followed by photocatalysis applied to the decontamination of water effluents from cellulose and paper factories. J. Hazard. Mater. 2008, 160, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-L.; Wang, C.-T.; Chang, S.-Y. Study of COD and turbidity removal from real oxide-CMP wastewater by iron electrocoagulation and the evaluation of specific energy consumption. J. Hazard. Mater. 2009, 168, 1200–1207. [Google Scholar] [CrossRef]
- Ge, J.; Qu, J.; Lei, P.; Liu, H. New bipolar electrocoagulation–electroflotation process for the treatment of laundry wastewater. Sep. Purif. Technol. 2004, 36, 33–39. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Fluoride removal by a continuous flow electrocoagulation reactor. J. Environ. Manag. 2009, 90, 1204–1212. [Google Scholar] [CrossRef]
- Bansal, S.; Kushwaha, J.P.; Sangal, V.K. Electrochemical Treatment of Reactive Black 5 Textile Wastewater: Optimization, Kinetics, and Disposal Study. Water Environ. Res 2013, 85, 2294–2306. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Lo, S.-L.; Liou, Y.-H.; Hu, C.-Y. Removal of nonsteroidal anti-inflammatory drugs (NSAIDs) by electrocoagulation–flotation with a cationic surfactant. Sep. Purif. Technol. 2015, 152, 148–154. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Hu, C.-Y.; Lo, S.-L. Direct and indirect electrochemical oxidation of amine-containing pharmaceuticals using graphite electrodes. J. Hazard. Mater. 2019, 366, 592–605. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M. A ligand based innovative composite material for selective lead(II) capturing from wastewater. J. Mol. Liq. 2019, 294, 111679. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Rahman, M.M.; Asiri, A.M. Novel composite material for selective copper(II) detection and removal from aqueous media. J. Mol. Liq. 2019, 283, 772–780. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Anglada, Á.; Urtiaga, A.; Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biot. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Qu, J. Electrocoagulation in Water Treatment. In Electrochemistry for the Environment; Comninellis, C., Chen, G., Eds.; Springer: New York, NY, USA, 2010; pp. 245–262. [Google Scholar] [CrossRef]
- Rychen, P.; Provent, C.; Pupunat, L.; Hermant, N. Domestic and Industrial Water Disinfection Using Boron-Doped Diamond Electrodes. In Electrochemistry for the Environment; Comninellis, C., Chen, G., Eds.; Springer: New York, NY, USA, 2010; pp. 143–161. [Google Scholar] [CrossRef]
- Pulkka, S.; Martikainen, M.; Bhatnagar, A.; Sillanpää, M. Electrochemical methods for the removal of anionic contaminants from water—A review. Sep. Purif. Technol. 2014, 132, 252–271. [Google Scholar] [CrossRef]
- Griesbach, U.; Malkowsky, I.M.; Waldvogel, S.R. Green Electroorganic Synthesis Using BDD Electrodes. In Electrochemistry for the Environment; Comninellis, C., Chen, G., Eds.; Springer: New York, NY, USA, 2010; pp. 125–141. [Google Scholar] [CrossRef]
- Steckhan, E.; Arns, T.; Heineman, W.R.; Hilt, G.; Hoormann, D.; Jörissen, J.; Kröner, L.; Lewall, B.; Pütter, H. Environmental protection and economization of resources by electroorganic and electroenzymatic syntheses. Chemosphere 2001, 43, 63–73. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Cabot, P.L.; Brillas, E.; Sirés, I. A comprehensive study on the electrochemical advanced oxidation of antihypertensive captopril in different cells and aqueous matrices. Appl. Catal. B Environ. 2020, 277, 119240. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Yue, P.L. Investigation on the electrolysis voltage of electrocoagulation. Chem. Eng. Sci. 2002, 57, 2449–2455. [Google Scholar] [CrossRef]
- He, C.-C.; Hu, C.-Y.; Lo, S.-L. Integrating chloride addition and ultrasonic processing with electrocoagulation to remove passivation layers and enhance phosphate removal. Sep. Purif. Technol. 2018, 201, 148–155. [Google Scholar] [CrossRef]
- Teixeira, S.; Delerue-Matos, C.; Alves, A.; Santos, L. Fast screening procedure for antibiotics in wastewaters by direct HPLC-DAD analysis. J. Sep. Sci. 2008, 31, 2924–2931. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-L.; Wang, C.-T.; Huang, K.-Y.; Liu, T.-C. Electrochemical removal of salicylic acid from aqueous solutions using aluminum electrodes. Desalination 2011, 271, 55–61. [Google Scholar] [CrossRef]
- Zhang, G.H.; Yin, L.L.; Zhang, S.T.; Li, X. Adsorption Behavior of Sulfamethoxazole as Inhibitor for Mild Steel in 3% HCl Solution. Adv. Mater. Res. 2011, 194–196, 8–15. [Google Scholar] [CrossRef]
- Ren, M.; Song, Y.; Xiao, S.; Zeng, P.; Peng, J. Treatment of berberine hydrochloride wastewater by using pulse electro-coagulation process with Fe electrode. Chem. Eng. J. 2011, 169, 84–90. [Google Scholar] [CrossRef]
- Indermuhle, C.; Martín de Vidales, M.J.; Sáez, C.; Robles, J.; Cañizares, P.; García-Reyes, J.F.; Molina-Díaz, A.; Comninellis, C.; Rodrigo, M.A. Degradation of caffeine by conductive diamond electrochemical oxidation. Chemosphere 2013, 93, 1720–1725. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Z.; Xu, Y.; Tian, J.; Qi, H.; Lin, W.; Cui, F. Oxidation of sulfamethoxazole (SMX) by chlorine, ozone and permanganate—A comparative study. J. Hazard. Mater. 2014, 274, 258–269. [Google Scholar] [CrossRef]
- Martins, A.F.; Mallmann, C.A.; Arsand, D.R.; Mayer, F.M.; Brenner, C.G.B. Occurrence of the Antimicrobials Sulfamethoxazole and Trimethoprim in Hospital Effluent and Study of Their Degradation Products after Electrocoagulation. CLEAN Soil Air Water 2011, 39, 21–27. [Google Scholar] [CrossRef]






| Categories | Compound (CAS Number) | Structure | MW (g mol−1) | Solubility (mg/L) | pKa | Log Kow |
|---|---|---|---|---|---|---|
| β-blocker | Atenolol (ATE) (29122-68-7) |  | 266.34 | 300 | 9.6 | 0.16 |
| Nonsteroidal anti-inflammatory drug (NSAID) | Diclofenac (DIC) (15307-86-5) |  | 296.13 | 2.37 | 4.15 | 4.51 |
| Sulfonamide antibiotic | Sulfamethoxazole (SMX) (723-46-6) |  | 253.28 | 370 | 5.7 | 0.89 |
| Time (min) | Mobile Phase | |
|---|---|---|
| A KH2PO4 (%) | B Acetonitrile (%) | |
| 0 | 80 | 20 |
| 3 | 80 | 20 |
| 4 | 60 | 40 |
| 10.5 | 60 | 40 |
| 11.5 | 40 | 60 |
| 18.5 | 40 | 60 |
| 19.5 | 80 | 20 |
| Parameters | Hospital Effluent |
|---|---|
| Temperature (°C) | 25.0 |
| Dissolved oxygen (DO) (ppm) | 3.35 |
| pH | 7.20 |
| Turbidity (NTU) | 10.9 |
| Suspended solids (SS) (mg/L) | 0.018 |
| Chemical oxygen demand (COD) (mg/L) | 41.5 |
| Biochemical oxygen demand (BOD) (mg/L) | 4.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-J.; Huang, Y.-L.; Lo, S.-L.; Hu, C.-Y. Comparing the Effects of Types of Electrode on the Removal of Multiple Pharmaceuticals from Water by Electrochemical Methods. Water 2020, 12, 2332. https://doi.org/10.3390/w12092332
Liu Y-J, Huang Y-L, Lo S-L, Hu C-Y. Comparing the Effects of Types of Electrode on the Removal of Multiple Pharmaceuticals from Water by Electrochemical Methods. Water. 2020; 12(9):2332. https://doi.org/10.3390/w12092332
Chicago/Turabian StyleLiu, Yu-Jung, Yung-Ling Huang, Shang-Lien Lo, and Ching-Yao Hu. 2020. "Comparing the Effects of Types of Electrode on the Removal of Multiple Pharmaceuticals from Water by Electrochemical Methods" Water 12, no. 9: 2332. https://doi.org/10.3390/w12092332
APA StyleLiu, Y. -J., Huang, Y. -L., Lo, S. -L., & Hu, C. -Y. (2020). Comparing the Effects of Types of Electrode on the Removal of Multiple Pharmaceuticals from Water by Electrochemical Methods. Water, 12(9), 2332. https://doi.org/10.3390/w12092332