Formation of Clay-Rich Layers at The Slip Surface of Slope Instabilities: The Role of Groundwater
Abstract
:1. Introduction
2. Geological and Geographical Setting of the Diezma Landslide
3. Characteristics of the Diezma Landslide
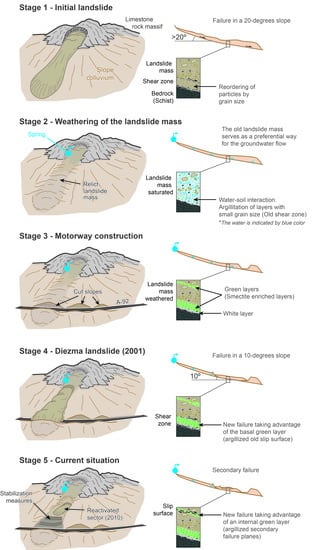
- The landslide head is located on the old Granada–Almería road (CN-342). In this area, several meter-scale scarps were observed. These scarps correspond to the shallow rotational slides that have developed successively on the clay-rich rocks of the flysch formation. The impermeable characteristic of these shear surfaces favored the development of ponds at the foot of the main scarp [11].
- The intermediate part of the landslide was formed by progressive rotational slides that produced some secondary scarps, which generated bulges with tension cracks at their crests.
- The landslide style grades downhill from a multiple rotational slide into a proper earthflow. In the toe sector, the thickness of the mass movement in the central area is approximately 30 m.
4. Methodology
4.1. Sampling Procedure
4.2. Mineralogical Characterization: X-Ray Diffractometry (XRD) and X-Ray Fluorescence (XRF)
4.3. Geophysical Survey: 2D Electrical Resistivity Tomography (ERT) Profiles
4.4. Geochemical Interpretative Methods: Ion-Ion Plots and Geochemical Modelling
5. Results
5.1. Mineralogical Characteristics of the Slip Zone
5.2. Internal Geometry and Groundwater Flow in the Diezma Landslide
5.3. Groundwater Hydrochemistry in the Diezma Landslide
5.4. Process Quantification by Mass-Balance Calculations
5.5. Direct modelling: Reaction-Path Calculations
6. Discussion
6.1. Where Does the Clay-Bearing Layer of the Diezma Landslide Come From?
6.2. Conceptual Model Proposed for the Evolution of the Diezma Landslide
- -
- The Diezma landslide mobilized surficial deposit most likely produced by a former mass movement (see Section 3).
- -
- Clay-bearing layers enriched with smectite appear associated to slip surfaces (see Section 3).
- -
- Smectite are high-plasticity clays that give the materials very low residual friction angles (ϕr = 7°). This allows occurrence of landslides showing failure planes with very low inclination (<20 °) (see Section 3).
- -
- Bi-carbonate type waters infiltrated in the slope of Diezma landslide favored the transformation of kaolinite to smectite (see Section 5).
- -
- Old slip surfaces may represent preferential flow ways for groundwater (see Section 6.1).
- -
- Chemical reactions in slip surfaces can be enhanced because they are composed of fine particles (i.e., layers with highly reactive surface areas) (see Section 6.1).
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shuzui, H. Process of slip surface development and formation of slip surface clay in landslide in Tertiary volcanic rocks, Japan. Eng. Geol. 2001, 61, 199–219. [Google Scholar] [CrossRef]
- Anson, R.; Hawkins, A. Movement of the Soper’s Wood landslide on the Jurassic Fuller’s Earth, Bath, England. Bull Eng. Geol. Environ. 2002, 61, 325–345. [Google Scholar] [CrossRef]
- Conte, E.; Donato, A.; Pugliese, L.; Troncone, A. Analysis of the Maierato landslide (Calabria, Southern Italy). Landslides 2018, 15, 1935–1950. [Google Scholar] [CrossRef]
- Conte, E.; Pugliese, L.; Troncone, A. Post-failure analysis of the Maierato landslide using the material point method. Eng. Geol. 2020, 277. [Google Scholar] [CrossRef]
- Bogaard, T.; Guglielmi, Y.; Marc, V.; Emblanch, C.; Bertrand, C.; Mudry, J. Hydrogeochemistry in landslide research: A review. Bull. Société Géologique Fr. 2007, 178, 113–126. [Google Scholar] [CrossRef]
- L’Heureux, J.S.; Locat, A.; Leroueil, S.; Demers, D.; Locat, J. Landslides in Sensitive Clays. In Geoscience to Risk Management; Springer: Dordrecht, The Netherlands, 2014; p. 418. [Google Scholar]
- Di Maio, C.; Santoli, L.; Schiavone, P. Volume change behaviour of clays: The influence of mineral composition, pore fluid composition and stress state. Mech. Mater. 2004, 36, 435–445. [Google Scholar] [CrossRef]
- Alvarado, G.; Vega, E.; Chaves, J.; Vászquez, M. Los grandes deslizamientos (volcánicos y no volcánicos) tipo debris avalanche en Costa Rica. Rev. Geol. Amér. Cent. 2004, 30, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Waythomas, C.F.; Pierson, T.C.; Major, J.J.; Scott, W.E. Voluminous ice-rich and water-rich lahars generated during the 2009 eruption of Redoubt Volcano, Alaska. J. Volcanol. Geotherm. Res. 2013, 259, 389–413. [Google Scholar] [CrossRef]
- Ferrer, M.; Ayala-Carcedo, F. Landslides in Spain: Extent and Assessment of the Climatic Susceptibility. In Proceedings of the Symposium on Engineering Geology and the Environment Balkema, Rotterdam, The Netherlands, 23–27 June 1997; Marinos, P.G., Koukis, G.C., Tsiambaos, G.C., Stournaras, G.C., Eds.; pp. 625–631. [Google Scholar]
- Azañón, J.M.; Azor, A.; Yesares, J.; Tsige, M.; Mateos, R.M.; Nieto, F.; Delgado, J.; López-Chicano, M.; Martín, W.; Rodríguez-Fernández, J. Regional-scale high-plasticity clay-bearing formation as controlling factor on landslides in Southeast Spain. Geomorphology 2010, 120, 26–37. [Google Scholar] [CrossRef]
- Korup, O.; Clague, J.J.; Hermanns, R.L.; Hewitt, K.; Strom, A.L.; Weidinger, J.T. Giants landslides, topography, and erosion. Earth Planet. Sci. Lett. 2007, 261, 578–589. [Google Scholar] [CrossRef]
- Hancox, G.T. The 1979 Abbotsford Landslide, Dunedin, New Zealand; a retrospective look at its nature and causes. Landslides 2008, 5, 177–188. [Google Scholar] [CrossRef]
- Dahal, R.K.; Hasegawa, S.; Yamanaka, M.; Dhakal, S.; Bhandary, N.P.; Yatabe, R. Comparative analysis of contributing parameters for rainfall-triggered landslide in the Lesser Himalaya of Nepal. Env. Geol. 2009, 58, 567–586. [Google Scholar] [CrossRef]
- Lundström, M.; Larsson, R.; Dahlin, T. Mapping of quick clay formations using geotechnical and geophysical methods. Landslides 2009, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Van Den Eeckhaut, M.; Hervás, J.; Jaedicke, C.; Malet, J.P.; Montanarella, L.; Nadim, F. Statistical modelling of Europe-Wide landslide susceptibility using limited landslide inventory data. Landslides 2012, 9, 357–369. [Google Scholar] [CrossRef]
- Dang, K.; Sassa, K.; Fukuoka, H.; Sakai, N.; Sato, Y.; Takara, K.; Quang, L.H.; Duy-Loi, H.; Van-Tiem, P.; Duc-Ha, N. Mechanism of two rapid and long-runout landslides in the 16 April 2016 earthquake using a ring-shear apparatus and computer simulation (LS-RAPID). Landslides 2016, 13, 1525–1534. [Google Scholar] [CrossRef]
- Prior, B.D.; Ho, C. Coastal and mountain slope instability on the islands of St. Lucia and Barbados. Eng. Geol. 1972, 6, 1–18. [Google Scholar] [CrossRef]
- Egashira, K.; Gibo, S. Colloid-chemical and mineralogical differences of smectites take from argillized layers, both from within and outside the slip surfaces in the Kamenose landslide. Appl. Clay Sci. 1988, 3, 253–262. [Google Scholar] [CrossRef]
- Angeli, M.C.; Pasuto, A.; Silvano, S. Towards definition of slope instability behaviour in the Alverà mudslide (Cortina d’Ampezzo, Italy). Geomorphology 1999, 30, 201–211. [Google Scholar] [CrossRef]
- Wen, B.P.; Aydin, A.; Aydin, N.S. Geochemical characteristics of the slip zones in granitic saprolite and the implication in their origin and evolution. Environ. Geol. 2004, 47, 140–154. [Google Scholar] [CrossRef]
- Baoping, W.; Haiyang, C. Mineral Compositions and Elements Concentrations as Indicators for the Role of Groundwater in the Development of Landslide Slip Zones: A Case Study of Large-scale Landslides in the Three Gorges Area in China. Earth Sci. Front. 2007, 14, 98–106. [Google Scholar]
- Li, X.; Liao, Q.; Wang, S.; Liu, J.; Lee, S. On evaluating the stability of the Baiyian ancient landslide in the Three Gorges Reservoir area, Yangtze River: A geological history analysis. Environ. Geo. L 2008, 55, 1699–1711. [Google Scholar] [CrossRef]
- Tang, H.; Li, C.; Hu, X.; Su, A.; Wang, L.; Wu, Y.; Criss, R.; Xiong, C.; Li, Y. Evolution characteristics of the Huangtupo landslide based on in situ tunnelling and monitoring. Landslides 2015, 12, 511–521. [Google Scholar] [CrossRef]
- Jiang, J.; Xiang, W.; Rohn, J.; Zen, W.; Schleier, M. Research on water-rock (soil) interaction by dynamic tracing method for Huangtupo landslide, Three Gorges Reservoir, PR China. Environ. Earth. Sci. 2015, 74, 557–571. [Google Scholar] [CrossRef]
- Jian, W.; Wang, Z.; Yin, K. Mechanism of the Anlesi landslide in the Three Gorges Reservoir, China. Eng. Geol. 2009, 108, 86–95. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, H.; Ran, J.; Li, W.; Sun, X. Field monitoring of groundwater responses to heavy rainfalls and the early warning of the Kualiangzi landslide in Sichuan Basin, southwestern China. Landslides 2016, 13, 1555–1570. [Google Scholar] [CrossRef]
- Nieto, F.; Abad, I.; Azañón, J.M. Smectite quantification in sediments and soils by thermogravimetric analyses. Appl. Clay Sci. 2008, 38, 288–296. [Google Scholar] [CrossRef]
- Guglielmi, Y.; Bertrand, C.; Compagnon, F.; Follacci, J.P.; Mudry, J. Acquisition of water chemistry in a mobile fissured basement massif: Its role in the hydrogeological knowledge of the La Clapière landslide (Mercantour massif, southern Alps, France). J. Hydrol. 2000, 229, 138–148. [Google Scholar] [CrossRef]
- De Montety, V.; Marc, V.; Emblanch, C.; Malet, J.P.; Bertrand, C.; Maquaire, O.; Bogaard, T.A. Identifying the origin of groundwater and flow processes in complex landslides affecting black marls: Insights from a hydrochemical survey. Earth Surf. Process. Landf. 2007, 32, 32–48. [Google Scholar] [CrossRef]
- Cervi, F.; Ronchetti, F.; Martinelli, G.; Bogaard, T.A.; Corsini, A. Origin and assessment of deep groundwater inflow in the Ca’ Lita landslide using hydrochemistry and in situ monitoring. Hydrol. Earth Syst. Sci. 2012, 16, 4205–4221. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Peces, M.J.; Azañón, J.M.; García-Mayordomo, J.; Yesares, J.; Troncoso, E.; Tsige, M. The Diezma landslide (A-92 motorway, Southern Spain): History and potential for future reactivation. Bull. Eng. Geol. Environ. 2011, 70, 681–689. [Google Scholar] [CrossRef]
- Delgado, J.; Garrido, J.; Lenti, L.; Lopez-Casado, C.; Martino, S.; Sierra, F.J. Unconventional pseudostatic stability analysis of the Diezma landslide (Granada, Spain) based on a high-resolution engineering-geological model. Eng. Geol. 2015, 184, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Bourgois, J.; Chauve, P.; Didon, J. La formation d’argiles a blocs dans la province de Cadix, Cordilleras Betiques, Espagne. Reun. Annu. Sci. Terre 1974, 2, 79. [Google Scholar]
- García-Dueñas, V.; Navarro-Vilá, F. Mapa Geológico de España E. 1:50.000, La Peza; I.G.M.E.: Madrid, Spain, 1980. [Google Scholar]
- Diputación Provincial de Granada e Instituto Tecnologico Geominero de España. Atlas Hidrogeológico de la Provincia de Granada; Diputación de Granada e ITGE: Granada, Spain, 1990; p. 107. [Google Scholar]
- Oteo-Mazo, C. Informe Sobre el Deslizamiento de Diezma (A-92) y las Soluciones para Estabilizarlo; Consejería de Obras Públicas y Urbanismo de la Junta de Andalucía: Madrid, Spain, 2001; p. 60. [Google Scholar]
- Delgado, J.; Garrido, J.; Lopez-Casado, C.; Lenti, L.; Martino, S.; Sierra, F.J. Diezma Landslide (Southern Spain): Geological Model and Seismic Response. Eng. Geol. Soc. Territ. 2015, 5. [Google Scholar] [CrossRef]
- Yesares-García, J.; Arocha, J.; Azañón, J.M.; Azor, A.; Díaz-Losada, E.; López-Chicano, M.; Martin, W.; Nieto, F.; Rodríguez-Fernández, J.; Garrido-Manrique, J. Factores Condicionantes en el Deslizamiento de Diezma (Granada, España). In Riesgos Naturales y Antropicos en Geomorfologia (Actas de la VIII Reunion Nacional de Geomorfologia); Benito, G., Díez-Herrero, A., Eds.; SEG y CSIG: Madrid, Spain, 2004; pp. 445–450. [Google Scholar]
- Oteo-Mazo, C. Diseño y ejecución del tratamiento para estabilizar el deslizamiento de Diezma (Granada). Spec. Vol. Congr. Andal. Carret. 2003, 3, 40–52. [Google Scholar]
- Azañón, J.M.; Azor, A.; Cardenal-Escarcena, J.F.; Delgado-García, J.; Delgado-Marchal, J.; Gómez-Molina, A.; López-Chicano, M.; López-Sánchez, J.M.; Mallorquí-Franquet, J.J.; Martín, W.; et al. Estudio Sobre la Predicción y Mitigación de Movimientos de Ladera en Vías de Comunicación Estratégicas de la Junta de Andalucía. Informe Final; Instituto Andaluz de Ciencias de la Tierra: Granada, Spain, 2006; p. 380. [Google Scholar]
- Azañón, J.M.; Abellán, A.; García, J.P.; Mateos, R.; Roldán, F.; Peña, J.P.; Galve, J.P.; Notti, D.; Monserrat, O.; Jover, R.T.; et al. Uso de Datos LIDAR Aéreos en 3D para el Control de Movimientos de Ladera. Caso de Estudio del Deslizamiento de Diezma (Granada). In Teledetección: Humedales y Espacios Protegidos. XVI Congreso de la Asociación Española de Teledetección; Bustamante, J., Díaz-Delgado, R., Aragonés, D., Afán, I., García, D., Eds.; Asociación Española de Teledetección: Sevilla, Spain, 2015; pp. 407–410. [Google Scholar]
- Umar, M.; Kassim, K.A.; Ping Chiet, K.T. Biological process of soil improvement in civil engineering: A review. J. Rock Mech. Geotech. Eng. 2016, 8, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Martín-Ramos, J.D. Using XPowder: A Software Package for Powder X-Ray Diffraction Analysis; 2004; GR 1001/04; ISBN 84-609-1497-6, Spain. Available online: http://www.xpowder.com (accessed on 18 September 2020).
- Azañón, J.M.; Peña, J.A.; Teixidó, T.; Mateos, R.M.; Yesares, J.; Delgado, J.; Tsiege, M. Evaluación de la eficacia de los sistemas de drenaje mediante tomografía eléctrica en el deslizamiento de Diezma (Granada). In Proceedings of the VII Simposio Nacional sobre Taludes y Laderas Inestables, Barcelona, Spain, 27–30 October 2009. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Techniques and Methods. In Description of Input and Examples for PHREEQC Version 3. A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 2013; Book 6, Chapter A43. [Google Scholar]
- Ball, J.W.; Nordstrom, D.K. WATEQ4F User’s Manual with Revised Thermodynamic Data Base and Test Cases for Calculating Speciation of Major, Trace and Redox Elements in Natural Waters; Open-File Report 90 129; U.S. Geological Survey: Reston, VA, USA, 2001.
- Subramani, T.; Rajmohan, N.; Elango, L. Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ. Monit. Assess. 2010, 162, 123–137. [Google Scholar] [CrossRef]
- Barberá, J.A.; Andreo, B.; Almeida, C. Using non-conservative tracers to characterise karstification processes in the Merinos-Colorado-Carrasco carbonate aquifer system (southern Spain). Environ. Earth. Sci. 2014, 71, 585–599. [Google Scholar] [CrossRef]
- Acero, P.; Gutiérrez, F.; Galve, J.P.; Auqué, L.F.; Carbonel, D.; Gimeno, M.J.; Gómez, J.B.; Asta, M.P.; Yechieli, Y. Hydrogeochemical characterization of an evaporite karst area affected by sinkholes (Ebro Valley, NE Spain). Geol. Acta 2013, 11, 389–407. [Google Scholar]
- Ledesma-Ruiz, R.; Pastén-Zapata, E.; Parra, R.; Harter, T.; Mahlknecht, J. Investigation of the geochemical evolution of groundwater under agricultural land: A case study in northeastern Mexico. J. Hydrol. 2015, 521, 410–423. [Google Scholar] [CrossRef] [Green Version]
- Garrels, R.M.; Mackenzie, F.T. Evolution of Sedimentary Rocks; W. W. Norton & Co.: New York, NY, USA, 1971; p. 397. [Google Scholar] [CrossRef]
- Holland, H.D. The Chemistry of the Atmosphere and Ocean; Wiley: New York, NY, USA, 1978; p. 351. [Google Scholar]
- Mayo, A.L.; Loucks, M.D. Solute and isotopic geochemistry and groundwater flow in the Central Wasatch-Range, Utah. J. Hydrol. 1995, 172, 31–59. [Google Scholar] [CrossRef]
- Katz, B.G.; Gopalan, T.B.; Bullen, T.D.; Davis, J.H. Use of chemical and isotopic tracers to characterize the interaction between groundwater and surface water in mantled Karst. Groundw. J. 1998, 35, 1014–1028. [Google Scholar] [CrossRef]
- Merkel, B.J.; Planer-Friedrich, B.; Nordstrom, D.K. Groundwater Geochemistry—A Practical Guide to Modeling of Natural and Contaminated Aquatic Systems; Springer: Berlin/Heidelberg, Germany, 2005; p. 200. [Google Scholar]
- Herman, J.S.; White, W.B. Dissolution kinetics of dolomite. Effects of lithology and fluid flow velocity. Geochim. Cosmochim. Acta 1985, 49, 2017–2026. [Google Scholar] [CrossRef]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice-Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Liu, Z.; Yuan, D.; Dreybrot, W. Comparative study of dissolution rate determinating mechanisms of limestone and dolomite. Environ. Geol. 2005, 49, 274–279. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1982; p. 388. [Google Scholar] [CrossRef]
- Tardy, Y. Characterization of the Principal Weathering Types by the Geochemistry of Waters from Some European and African Crystalline Massifs. Chem. Geol. 1971, 7, 253–271. [Google Scholar] [CrossRef]
- Norton, D. Chemical mass transfer in the Rio Tanama system, west-central Puerto Rico. Geochim. Cosmochim. Acta 1974, 38, 267–277. [Google Scholar] [CrossRef]
- Garrels, R.M.; Mackenzie, F.T. Origin of the Chemical Composition of Some Springs and Systems. In Equilibrium Concepts in Natural Water Systems; American Chemical Society: Washington, DC, USA, 1967; Volume 67, pp. 222–242. [Google Scholar]
- Auqué, L.F.; Acero, P.; Gimeno, M.J.; Gómez, J.B.; Asta, M.P. Hydrogeochemical modeling of a thermal system and lessons learned for CO2 geologic storage. Chem. Geol. 2009, 268, 324–336. [Google Scholar] [CrossRef]
- Acero, P.; Auqué, L.F.; Galve, J.P.; Gutiérrez, F.; Carbonel, D.; Gimeno, M.J.; Gómez, J.B.; Asta, M.P.; Yechieli, Y. Evaluation of geochemical and hydrogeological processes by geochemical modeling in an area affected by evaporite karstification. J. Hydrol. 2015, 529, 1874–1889. [Google Scholar] [CrossRef]
- Asta, M.P.; Calleja, M.L.L.; Pérez-López, R.; Auqué, L.F. Major hydrogeochemical processes in an Acid Mine Drainage affected estuary. Mar. Pollut. Bull. 2015, 91, 295–305. [Google Scholar] [CrossRef]
- Zhu, C.; Anderson, G. Environmental Applications of Geochemical Modelling; Cambridge University Press: Cambridge, UK, 2002; p. 284. [Google Scholar]
- Polyak, V.J.; Güven, N. Authigenesis of trioctahedral smectite in magnesium-rich carbonate speleothems in Carlsbad Cavern and other caves of the Guadalupe Mountains, New Mexico. Clays Clay Miner. 2000, 48, 317–321. [Google Scholar] [CrossRef]
- Nagai, S. Understanding of groundwater flow by water analysis—Groundwater in Maebashi, Takasaki and Isesaki regions. Ind. Waters 1968, 114, 66–75. (In Japanese) [Google Scholar]
- Fukuoka, H.; Sassa, K.; Wang, G. Influence of shear speed and normal stress on the shear. Landslides 2007, 4, 63–74. [Google Scholar] [CrossRef]
- Sassa, K.; Fukuoka, H.; Wang, G.; Ishikawa, N. Undrained dynamic-loading ring-shear apparatus and its application to landslide dynamics. Landslides 2004, 1, 7–19. [Google Scholar] [CrossRef]
- Abad, I.; Jiménez-Millán, J.; Schleicher, A.M.; Van Der Pluijm, B.A. Mineral characterization, clay quantification and Ar-Ar dating of faulted schists in the Carboneras and Palomares Faults (Betic Cordillera, SE Spain). Eur. J. Mineral. 2017, 29, 17–34. [Google Scholar] [CrossRef]













| Parameters | Landslide Mass | Landslide Slip Surface |
|---|---|---|
| Water Content (%) | 15 | 30 |
| Dry density (g/cm3) | 1.7–1.8 | 1.42–1.59 |
| Natural density (g/cm3) | 2.1 | 1.8 |
| % Fine grained (<0.074 mm) | 84–95 | 98–99 |
| Specific gravity (G) | 2.44 | 2.24 |
| Void ratio (e) | 0.47 | 0.66 |
| Porosity (%) | 31 | 40 |
| Saturation (S) | >90 | >88 |
| Limit liquid (%) | 46 | 80 |
| Plasticity index (%) | 29 | 56 |
| Liquidity Index | −0.06 (HOC) | 0.1 (LOC) |
| Classification (USCS) | CH-MH | CH |
| % CaCO3 | 35 | 4–15 |
| Clay mineral composition | Smectite + illite | Smectite (>90%) |
| Swelling pressure (kPa) | 200 | 450 |
| φ’ (pick)/residual | 34–36°/20 | 19–20/7 |
| C’ (Kpa) | 20 | 39 |
| Cc | 0.01 | 0.02 |
| Cs | 0.0065 | 0.006 |
| Compound | (%) | Element | Ppm |
|---|---|---|---|
| SiO2 | 64.23 | O | 527,300 |
| Al2O3 | 13.15 | Si | 300,300 |
| H2O | 9.63 | Al | 69,610 |
| Fe2O3 | 6.05 | Fe | 42,320 |
| K2O | 2.925 | K | 24,280 |
| MgO | 2.121 | Mg | 12,790 |
| CaO | 0.814 | H | 10,780 |
| TiO2 | 0.665 | Ca | 5820 |
| Na2O | 0.197 | Ti | 3980 |
| P2O5 | 0.068 | Na | 1460 |
| MnO | 0.0308 | P | 300 |
| SO3 | 0.02 | Mn | 238 |
| Cr2O3 | 0.019 | Cr | 135 |
| CuO | 0.0153 | Cu | 122 |
| ZnO | 0.014 | Rb | 122 |
| Rb2O | 0.0133 | Zn | 113 |
| SrO | 0.00922 | S | 81 |
| NiO | 0.00907 | Sr | 78 |
| Ga2O3 | 0.0026 | Ni | 71 |
| Y2O3 | 0.00202 | Ga | 19 |
| Y | 16 |
| Date | Sample | pH | Ca | K | Mg | Na | SiO2 | SO4 | Cl | HCO3− | Ca/Mg | TDS | Saturation Indices | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mmol/L) | (mg/L) | Cal | Dol | Gy | Anh | Hal | |||||||||||
| October 11 | P1 | 7.77 | 2.05 | 0.01 | 0.33 | 0.17 | n.m. | 0.04 | 0.22 | 4.55 | 6.2 | 385 | 0.66 | 0.67 | −2.95 | −3.17 | −9.09 |
| P2 | 8.17 | 1.51 | 0.01 | 0.58 | 0.30 | n.m. | 0.45 | 0.33 | 3.39 | 2.6 | 339 | 0.79 | 1.29 | −1.99 | −2.21 | −8.67 | |
| P3 | 8.09 | 1.75 | 0.01 | 0.82 | 0.38 | n.m. | 0.36 | 0.43 | 4.15 | 2.1 | 397 | 0.85 | 1.50 | −2.07 | −2.29 | −8.45 | |
| February 13 | P1 | 7.56 | 2.08 | 0.00 | 0.53 | 0.12 | n.m. | 0.08 | 0.15 | 5.00 | 3.9 | 427 | 0.50 | 0.54 | −2.63 | −2.85 | −9.40 |
| P2 | 7.89 | 2.53 | 0.02 | 0.70 | 0.22 | n.m. | 0.37 | 0.26 | 5.95 | 3.6 | 529 | 0.94 | 1.46 | −1.94 | −2.16 | −8.92 | |
| P3 | 8.02 | 1.72 | 0.02 | 0.84 | 0.38 | n.m. | 0.49 | 0.30 | 4.40 | 2.1 | 421 | 0.95 | 1.47 | −1.93 | −2.15 | −8.92 | |
| November 14 | P1 | 7.85 | 1.88 | 0.00 | 0.56 | 0.21 | 0.32 | 0.06 | 0.27 | 4.75 | 3.4 | 398 | 0.72 | 1.04 | −2.82 | −3.04 | −8.92 |
| P2 | 7.91 | 2.23 | 0.03 | 0.72 | 0.32 | 0.48 | 0.44 | 0.32 | 5.00 | 3.1 | 473 | 0.84 | 1.33 | −1.90 | −2.12 | −8.66 | |
| P3 | 7.90 | 1.80 | 0.00 | 1.11 | 0.50 | 0.80 | 0.30 | 0.70 | 5.10 | 1.6 | 473 | 0.75 | 1.43 | −2.16 | −2.38 | −8.12 | |
| Year | Sampling Point | Type of Data | pH | Ca | K | Mg | Na | SiO2 | SO4 | Cl | HCO3− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mmol/L | |||||||||||
| 2011 | P2 | Measured | 8.17 | 1.51 | 0.01 | 0.58 | 0.30 | n.m. | 0.45 | 0.33 | 3.39 |
| Calculated | 8.14 | 1.60 | 0.01 | 0.58 | 0.30 | - | 0.45 | 0.33 | 3.39 | ||
| P3 | Measured | 8.09 | 1.75 | 0.01 | 0.82 | 0.38 | n.m. | 0.36 | 0.43 | 4.15 | |
| Calculated | 8.10 | 1.59 | 0.01 | 0.82 | 0.38 | - | 0.36 | 0.41 | 4.25 | ||
| 2013 | P2 | Measured | 7.89 | 2.53 | 0.02 | 0.70 | 0.22 | n.m. | 0.37 | 0.26 | 5.95 |
| Calculated | 7.88 | 2.69 | 0.02 | 0.70 | 0.22 | - | 0.37 | 0.26 | 5.95 | ||
| P3 | Measured | 8.02 | 1.72 | 0.02 | 0.84 | 0.38 | n.m. | 0.49 | 0.30 | 4.40 | |
| Calculated | 8.05 | 1.66 | 0.02 | 0.84 | 0.42 | - | 0.49 | 0.30 | 4.39 | ||
| 2014 | P2 | Measured | 7.91 | 2.23 | 0.03 | 0.72 | 0.32 | 0.48 | 0.44 | 0.32 | 5.00 |
| Calculated | 7.91 | 2.20 | 0.01 | 0.72 | 0.32 | 0.48 | 0.44 | 0.32 | 5.00 | ||
| P3 | Measured | 7.90 | 1.80 | 0.00 | 1.11 | 0.50 | 0.80 | 0.30 | 0.70 | 5.10 | |
| Calculated | 7.90 | 1.85 | 0.03 | 1.11 | 0.50 | 0.80 | 0.30 | 0.70 | 5.10 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, J.; Asta, M.P.; Galve, J.P.; Azañón, J.M. Formation of Clay-Rich Layers at The Slip Surface of Slope Instabilities: The Role of Groundwater. Water 2020, 12, 2639. https://doi.org/10.3390/w12092639
Castro J, Asta MP, Galve JP, Azañón JM. Formation of Clay-Rich Layers at The Slip Surface of Slope Instabilities: The Role of Groundwater. Water. 2020; 12(9):2639. https://doi.org/10.3390/w12092639
Chicago/Turabian StyleCastro, Julia, Maria P. Asta, Jorge P. Galve, and José Miguel Azañón. 2020. "Formation of Clay-Rich Layers at The Slip Surface of Slope Instabilities: The Role of Groundwater" Water 12, no. 9: 2639. https://doi.org/10.3390/w12092639
APA StyleCastro, J., Asta, M. P., Galve, J. P., & Azañón, J. M. (2020). Formation of Clay-Rich Layers at The Slip Surface of Slope Instabilities: The Role of Groundwater. Water, 12(9), 2639. https://doi.org/10.3390/w12092639