Enhanced Degradation of Rhodamine B through Peroxymonosulfate Activated by a Metal Oxide/Carbon Nitride Composite
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Catalyst
2.3. Degradation Experiment
2.4. Characterizations
2.5. Analytical Methods
3. Result and Discussion
3.1. Degradation of RhB in Different Systems
3.2. Effect of Preparation Procedures on the Catalytic Performance of CM/g-C3N4
3.3. Characterizations of the Catalysts
3.3.1. SEM and TEM
3.3.2. XRD
3.3.3. FTIR
3.3.4. BET
3.4. Factors Impacting RhB Degradation by CM/g-C3N4/PMS
3.4.1. Catalyst Dosage, PMS Concentration and RhB Concentration
3.4.2. pH
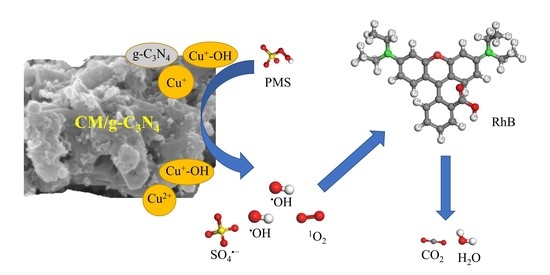
3.5. Possible Activation Mechanisms of PMS
3.6. Degradation Pathway of RhB and Toxicity Estimation
3.7. Stability of the CM/g-C3N4
3.8. Practical Application of the CM/g-C3N4/PMS System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solis, M.; Solis, A.; Ines Perez, H.; Manjarrez, N.; Flores, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar] [CrossRef]
- Ren, Q.; Nie, M.; Yang, L.; Wei, F.; Ding, B.; Chen, H.; Liu, Z.; Liang, Z. Synthesis of MOFs for RhB Adsorption from Wastewater. Inorganics 2022, 10, 27. [Google Scholar] [CrossRef]
- Sivarajasekar, N.; Baskar, R. Agriculture waste biomass valorisation for cationic dyes sequestration: A concise review. J. Chem. Pharm. Res. 2015, 7, 737–748. [Google Scholar]
- Shi, X.; Hong, P.; Huang, H.; Yang, D.; Zhang, K.; He, J.; Li, Y.; Wu, Z.; Xie, C.; Liu, J.; et al. Enhanced peroxymonosulfate activation by hierarchical porous Fe3O4/Co3S4 nanosheets for efficient elimination of rhodamine B: Mechanisms, degradation pathways and toxicological analysis. J. Colloid Interface Sci. 2022, 610, 751–765. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, S.; Li, X.; Du, Y.; Xing, Y.; Xu, Q.; Wang, Z.; Li, L.; Zhu, X. One-step pyrolysis for the preparation of sulfur-doped biochar loaded with iron nanoparticles as an effective peroxymonosulfate activator for RhB degradation. New J. Chem. 2022, 46, 5678–5689. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Decontamination of textile wastewater via TiO2/activated carbon composite materials. Adv. Colloid Interface Sci. 2010, 159, 130–143. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.A.; Talip, B.; Mohamed, R.; Kassim, A.H. Mycoremediation of Remazol Brilliant Blue R in greywater by a novel local strain of Aspergillus iizukae 605EAN: Optimisation and mechanism study. Int. J. Environ. Anal. Chem. 2020, 100, 1650–1668. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghanbari, D.; Salavati-Niasari1, M.; Hassanpour, M. Photo-degradation of organic dyes: Simple chemical synthesis of Ni(OH)2 nanoparticles, Ni/Ni(OH)2 and Ni/NiO magnetic nanocomposites. J. Mater. Sci.-Mater. Electron. 2016, 27, 1244–1253. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Y.; Liang, J.; Luo, Y.; Liu, Q.; Yang, Y.; Sun, X. Facile hydrothermal synthesis of co-glycerate as an efficient peroxymonosulfate activator for rhodamine B degradation. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 648, 129239. [Google Scholar] [CrossRef]
- Yousefi, S.; Alshamsi, H.; Amiri, O.; Salavati-Niasari, M. Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol. Liq. 2021, 337, 116405. [Google Scholar] [CrossRef]
- Syuhei, Y.; Kohei, M.; Hidenori, Y. Catalytic oxidation of benzene to phenol with hydrogen peroxide over Fe-terpyridine complexes supported on a cation exchange resin. Catal. Commun. 2018, 116, S1566736718303224. [Google Scholar]
- Amanollahi, H.; Moussavi, G.; Giannakis, S. Enhanced vacuum UV-based process (VUV/H2O2/PMS) for the effective removal of ammonia from water: Engineering configuration and mechanistic considerations. J. Hazard. Mater. 2021, 402, 123789. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Shao, Z.; Wang, S. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B Environ. 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Ling, S.K.; Wang, S.; Peng, Y. Oxidative degradation of dyes in water using Co2+/H2O2 and Co2+/peroxymonosulfate. J. Hazard. Mater. 2010, 178, 385–389. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Pacheco-Alvarez, M.O.A.; Wrobel, K.; Paramo-Vargas, J.; Bandala, E.R.; Brillas, E.; Peralta-Hernandez, J.M. Development of a Co2+/PMS process involving target contaminant degradation and PMS decomposition. Int. J. Environ. Sci. Technol. 2020, 17, 17–26. [Google Scholar] [CrossRef]
- Ulucan-Altuntas, K.; Guvenc, S.Y.; Can-Guven, E.; Ilhan, F.; Varank, G. Degradation of oxytetracycline in aqueous solution by heat-activated peroxydisulfate and peroxymonosulfate oxidation. Environ. Sci. Pollut. Res. 2022, 29, 9110–9123. [Google Scholar] [CrossRef]
- Huang, S.; Guo, X.; Duan, W.; Cheng, X.; Zhang, X.; Li, Z. Degradation of high molecular weight polyacrylamide by alkali-activated persulfate: Reactivity and potential application in filter cake removal before cementing. J. Pet. Sci. Eng. 2019, 174, 70–79. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Enhanced peroxymonosulfate activation for sulfamethazine degradation by ultrasound irradiation: Performances and mechanisms. Chem. Eng. J. 2018, 335, 145–153. [Google Scholar] [CrossRef]
- Cui, C.; Jin, L.; Jiang, L.; Han, Q.; Lin, K.; Lu, S.; Zhang, D.; Cao, G. Removal of trace level amounts of twelve sulfonamides from drinking water by UV-activated peroxymonosulfate. Sci. Total Environ. 2016, 572, 244–251. [Google Scholar] [CrossRef]
- Li, T.; Du, X.; Deng, J.; Qi, K.; Zhang, J.; Gao, L.; Yue, X. Efficient degradation of Rhodamine B by magnetically recoverable Fe3O4-modified ternary CoFeCu-layered double hydroxides via activating peroxymonosulfate. J. Environ. Sci. 2021, 108, 188–200. [Google Scholar] [CrossRef]
- Wan, Q.; Chen, Z.; Cao, R.; Wang, J.; Huang, T.; Wen, G.; Ma, J. Oxidation of organic compounds by PMS/CuO system: The significant discrepancy in borate and phosphate buffer. J. Clean. Prod. 2022, 339, 130773. [Google Scholar] [CrossRef]
- Deng, J.; Ya, C.; Ge, Y.; Cheng, Y.; Chen, Y.; Xu, M.; Wang, H. Activation of peroxymonosulfate by metal (Fe, Mn, Cu and Ni) doping ordered mesoporous Co3O4 for the degradation of enrofloxacin. Rsc Adv. 2018, 8, 2338–2349. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhang, J.; Zhou, H.; Yao, G.; Lai, B. Synergistic multiple active species for the degradation of sulfamethoxazole by peroxymonosulfate in the presence of CuO@FeOx@Fe-0. Chem. Eng. J. 2020, 380, 122568. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Zhang, D.; Lan, Y.; Guo, J. CuO-Co3O4@CeO2 as a heterogeneous catalyst for efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate. J. Hazard. Mater. 2020, 381, 122568. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Liu, Y.; Liu, P. Heterogeneous activation of peroxymonosulfate by Cu/ZSM5 for decolorization of Rhodamine B. Sep. Purif. Technol. 2014, 135, 1–6. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Si, F.; Yao, C.; Du, M.; Hussain, I.; Kim, H.; Huang, S.; Lin, Z.; Hayat, W. Persulfate non-radical activation by nano-CuO for efficient removal of chlorinated organic compounds: Reduced graphene oxide-assisted and CuO (001) facet-dependent. Chem. Eng. J. 2019, 356, 178–189. [Google Scholar] [CrossRef]
- Kiani, R.; Mirzaei, F.; Ghanbari, F.; Feizi, R.; Mehdipour, F. Real textile wastewater treatment by a sulfate radicals-Advanced Oxidation Process: Peroxydisulfate decomposition using copper oxide (CuO) supported onto activated carbon. J. Water Process Eng. 2020, 38, 101623. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Huang, W.; Wei, X.; Huang, W. Biochar supported CuO composites used as an efficient peroxymonosulfate activator for highly saline organic wastewater treatment. Sci. Total Environ. 2020, 721, 137764. [Google Scholar] [CrossRef]
- Yin, Z.; Han, M.; Hu, Z.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Peroxymonosulfate enhancing visible light photocatalytic degradation of bezafibrate by Pd/g-C3N4 catalysts: The role of sulfate radicals and hydroxyl radicals. Chem. Eng. J. 2020, 390, 124532. [Google Scholar] [CrossRef]
- Xu, M.; Han, L.; Dong, S. Facile Fabrication of Highly Efficient g-C3N4/Ag2O Heterostructured Photocatalysts with Enhanced Visible-Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2013, 5, 12533–12540. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, S.; Yu, J.S. Novel ordered nanoporous graphitic C3N4 as a support for Pt-Ru anode catalyst in direct methanol fuel cell. J. Mater. Chem. 2007, 17, 1656–1659. [Google Scholar] [CrossRef]
- Liu, S.; Wei, X.; Lin, S.; Guo, M. Preparation of aerogel Mg(OH)(2) nanosheets by a combined sol-gel-hydrothermal process and its calcined MgO towards enhanced degradation of paraoxon pollutants. J. Sol-Gel Sci. Technol. 2021, 99, 122–131. [Google Scholar] [CrossRef]
- Ali, J.; Jiang, W.; Shahzad, A.; Ifthikar, J.; Yang, X.; Wu, B.; Oyekunle, D.T.; Jia, W.; Chen, Z.; Zheng, L.; et al. Isolated copper ions and surface hydroxyl groups as a function of non-redox metals to modulate the reactivity and persulfate activation mechanism of spinel oxides. Chem. Eng. J. 2021, 425, 130679. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, Y.; Li, W.; Zhou, L.; Lan, Y.; LI, Y. Insight into heterogeneous catalytic degradation of sulfamethazine by peroxymonosulfate activated with CuCo2O4 derived from bimetallic oxalate. Chem. Eng. J. 2020, 384, 123257. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Yao, X.; Zhang, Y.; Wang, Y. A comprehensive insight into plasma-catalytic removal of antibiotic oxytetracycline based on graphene-TiO2-Fe3O4 nanocomposites. Chem. Eng. J. 2021, 425, 130614. [Google Scholar] [CrossRef]
- Yousefi, A.; Alireza, N. Photodegradation pathways of phenazopyridine by the CdS-WO3 hybrid system and its capability for the hydrogen generation. Mater. Res. Bull. 2022, 148, 111669. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Chen, X.; Webster, R.D.; Lim, T.-T. Facile synthesis of pure g-C3N4 materials for peroxymonosulfate activation to degrade bisphenol A: Effects of precursors and annealing ambience on catalytic oxidation. Chem. Eng. J. 2020, 387, 123726. [Google Scholar] [CrossRef]
- Song, H.; Liu, Z.; Guan, Z.; Yang, F.; Xia, D.; Li, D. Efficient persulfate non-radical activation of electron-rich copper active sites induced by oxygen on graphitic carbon nitride. Sci. Total Environ. 2021, 762, 143127. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Xu, X.; Cao, R.; Yang, H.; Xu, X.; Li, J. MOF-derived CoN/N-C@SiO2 yolk-shell nanoreactor with dual active sites for highly efficient catalytic advanced oxidation processes. Chem. Eng. J. 2020, 381, 122670. [Google Scholar] [CrossRef]
- Dan, J.; Rao, P.; Wang, Q.; Dong, L.; Chu, W.; Zhang, M.; He, Z.; Gao, N.; Deng, J.; Chen, J. MgO-supported CuO with encapsulated structure for enhanced peroxymonosulfate activation to remove thiamphenicol. Sep. Purif. Technol. 2022, 280, 119782. [Google Scholar] [CrossRef]
- Qui Thanh Hoai, T.; Namgung, G.; Noh, J.-S. Facile synthesis of porous metal-doped ZnO/g-C3N4 composites for highly efficient photocatalysts. J. Photochem. Photobiol. A Chem. 2019, 368, 110–119. [Google Scholar]
- Kang, Y.; Yang, Y.; Yin, L.-C.; Kang, X.; Liu, G.; Cheng, H.M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Huang, Z.-H.; Kang, F.; Yang, Q.H. Holey Graphitic Carbon Nitride Nanosheets with Carbon Vacancies for Highly Improved Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Lee, S.J.; Begildayeva, T.; Jung, H.J.; Koutavarapu, R.; Yu, Y.; Choi, M.; Choi, M.Y. Plasmonic ZnO/Au/g-C3N4 nanocomposites as solar light active photocatalysts for degradation of organic contaminants in wastewater. Chemosphere 2021, 263, 128262. [Google Scholar] [CrossRef]
- Song, H.; Guan, Z.; Xia, D.; Xu, H.; Yang, F.; Li, D.; Li, X. Copper-oxygen synergistic electronic reconstruction on g-C3N4 for efficient non-radical catalysis for peroxydisulfate and peroxymonosulfate. Sep. Purif. Technol. 2021, 257, 117957. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Lyu, L.; Zeng, Q.; Xing, X.; Hu, C. Electronic Structure Modulation of Graphitic Carbon Nitride by Oxygen Doping for Enhanced Catalytic Degradation of Organic Pollutants through Peroxymonosulfate Activation. Environ. Sci. Technol. 2018, 52, 14371–14380. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Ivanova, L.; Rosenholm, J.; Linden, M. Cobalt oxide species supported on SBA-15, KIT-5 and KIT-6 mesoporous silicas for ethyl acetate total oxidation. Appl. Catal. B Environ. 2009, 89, 365–374. [Google Scholar] [CrossRef]
- Wu, M.; Li, L.; Xue, Y.; Xu, G.; Tang, L.; Liu, N.; Huang, W.-Y. Fabrication of ternary GO/g-C3N4/MoS2 flower-like heterojunctions with enhanced photocatalytic activity for water remediation. Appl. Catal. B Environ. 2018, 228, 103–112. [Google Scholar] [CrossRef]
- Harish, S.; Archana, J.; Sabarinathan, M.; Navaneethan, M.; Nisha, K.D.; Ponnusamy, S.; Muthamizhchelvan, C.; Ikeda, H.; Aswal, D.K.; Hayakawa, Y. Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl. Surf. Sci. 2017, 418, 103–112. [Google Scholar] [CrossRef]
- Li, D.; Zan, J.; Wu, L.; Zuo, S.; Xia, D. Heterojunction tuning and catalytic efficiency of g-C3N4-Cu2O with glutamate. Ind. Eng. Chem. Res. 2019, 58, 4000–4009. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, G.; Wang, X. Synthesis of Carbon Nitride Semiconductors in Sulfur Flux for Water Photoredox Catalysis. ACS Catal. 2012, 2, 940–948. [Google Scholar] [CrossRef]
- Parvari, R.; Ghorbani-Shahna, F.; Bahrami, A.; Azizian, S.; Assari, M.J.; Farhadian, M. A novel core-shell structured alpha-Fe2O3/Cu/g-C3N4 nanocomposite for continuous photocatalytic removal of air ethylbenzene under visible light irradiation. J. Photochem. Photobiol. A Chem. 2020, 399, 112643. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Q.; Zhang, M.; Nie, Y.; Tian, X.; Yang, C.; Li, Y. pH-dependent oxidation mechanisms over FeCu doped g-C3N4 for ofloxacin degradation via the efficient peroxymonosulfate activation. J. Clean. Prod. 2021, 315, 128207. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Deng, H. Ag modified g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Mol. Catal. A Chem. 2016, 423, 270–276. [Google Scholar] [CrossRef]
- Zhu, J.N.; Zhu, X.Q.; Cheng, F.F.; Li, P.; Xiong, W.W. Preparing copper doped carbon nitride from melamine templated crystalline copper chloride for Fenton-like catalysis. Appl. Catal. B Environ. 2019, 256, 117830. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Yang, L.; Lan, Y. Degradation of methyl orange by sodium persulfate activated with zero-valent zinc. Sep. Purif. Technol. 2014, 132, 168–173. [Google Scholar] [CrossRef]
- Lu, H.; Sui, M.; Yuan, B.; Wang, J.; Lv, Y. Efficient degradation of nitrobenzene by Cu-Co-Fe-LDH catalyzed peroxymonosulfate to produce hydroxyl radicals. Chem. Eng. J. 2019, 357, 140–149. [Google Scholar] [CrossRef]
- Timmins, G.S.; Liu, K.J.; Bechara, E.J.H.; Kotake, Y.; Swartz, H.M. Trapping of free radicals with direct in vivo EPR detection: A comparison of 5,5-dimethyl-1-pyrroline-N-oxide and 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide as spin traps for HO. and SO4. Free Radic. Biol. Med. 1999, 27, 329–333. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Wu, S.; Yin, R.; Zhu, M. Surface dual redox cycles of Mn(III)/Mn(IV) and Cu(I)/Cu(II) for heterogeneous peroxymonosulfate activation to degrade diclofenac: Performance, mechanism and toxicity assessment. J. Hazard. Mater. 2021, 410, 124623. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.; Liu, W.; Xue, T.; Liu, C.; Zhang, Y.; Liu, L.; Wang, Q.; Qi, F.; Xu, B.; et al. Novel CuCo2O4 Composite Spinel with a Meso-Macroporous Nanosheet Structure for Sulfate Radical Formation and Benzophenone-4 Degradation: Interface Reaction, Degradation Pathway, and DFT Calculation. ACS Appl. Mater. Interfaces 2020, 12, 20522–20535. [Google Scholar] [CrossRef]
- Wang, R.; An, H.; Zhang, H.; Zhang, X.; Feng, J.; Wei, T.; Ren, Y. High active radicals induced from peroxymonosulfate by mixed crystal types of CuFeO2 as catalysts in the water. Appl. Surf. Sci. 2019, 484, 1118–1127. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Guo, J.; Zhou, L.; Lan, Y. Sulfur-doped copper-cobalt bimetallic oxides with abundant Cu(I): A novel peroxymonosulfate activator for chloramphenicol degradation. Chem. Eng. J. 2019, 361, 1304–1316. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Pan, Y.; Xiong, Z.; Yao, G.; Xie, R.; Lai, B. Peroxymonosulfate activation on FeCo2S4 modified g-C3N4 (FeCo2S4-CN): Mechanism of singlet oxygen evolution for nonradical efficient degradation of sulfamethoxazole. Chem. Eng. J. 2020, 384, 123361. [Google Scholar] [CrossRef]
- Wu, S.; Liang, G.; Guan, X.; Qian, G.; He, Z. Precise control of iron activating persulfate by current generation in an electrochemical membrane reactor. Environ. Int. 2019, 131, 105024. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Liao, H.; Liu, X.; Zhou, L.; Lan, Y.; Zhang, J. Enhanced degradation of 2,4,6-trichlorophenol by activated peroxymonosulfate with sulfur doped copper manganese bimetallic oxides. Chem. Eng. J. 2021, 417, 128121. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.-M.; Yang, X.-F.; Jiao, M.-G.; Zhou, Z.; Zhang, M.-H.; Wang, D.-H.; Bu, X.-H. Electronic structure of heterojunction MoO2/g-C3N4 catalyst for oxidative desulfurization. Appl. Catal. B Environ. 2018, 238, 263–273. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Lu, S.; Su, L.; Wang, C.; Huang, J.; Zhou, J.; Tang, J.; Huang, M. Nano-porous bimetallic CuCo-MOF-74 with coordinatively unsaturated metal sites for peroxymonosulfate activation to eliminate organic pollutants: Performance and mechanism. Chemosphere 2021, 273, 129643. [Google Scholar] [CrossRef]
- Wagner, G.W.; Yang, Y.C. Rapid nucleophilic/oxidative decontamination of chemical warfare agents. Ind. Eng. Chem. Res. 2002, 41, 1925–1928. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Chen, L.; Cai, T. Degradation of norfloxacin by CoFe alloy nanoparticles encapsulated in nitrogen doped graphitic carbon (CoFe@N-GC) activated peroxymonosulfate. Chem. Eng. J. 2020, 392, 123725. [Google Scholar] [CrossRef]
- Oh, W.-D.; Chang, V.W.C.; Hu, Z.-T.; Goei, R.; Lim, T.-T. Enhancing the catalytic activity of g-C3N4 through Me doping (Me=Cu, Co and Fe) for selective sulfathiazole degradation via redox-based advanced oxidation process. Chem. Eng. J. 2017, 323, 260–269. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Tian, J.; Yang, F.; Duan, X.; Gao, S.; Shi, W.; Luo, X.; Cui, F.; Luo, S.; Wang, S. Identification and Regulation of Active Sites on Nanodiamonds: Establishing a Highly Efficient Catalytic System for Oxidation of Organic Contaminants. Adv. Funct. Mater. 2018, 28, 1705295. [Google Scholar] [CrossRef]
- Rasalingam, S.; Peng, R.; Koodali, R.T. An insight into the adsorption and photocatalytic degradation of rhodamine B in periodic mesoporous materials. Appl. Catal. B Environ. 2015, 174, 49–59. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Zhou, P.; Li, W.; Zhang, J.; Zhang, G.; Cheng, X.; Liu, Y.; Huo, X.; Zhang, Y. Removal of Rhodamine B during the corrosion of zero valent tungsten via a tungsten species-catalyzed Fenton-like system. J. Taiwan Inst. Chem. Eng. 2019, 100, 202–209. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Karthikeyan, P.; Park, C.M.; Meenakshi, S. Boosted insights of novel accordion-like (2D/2D) hybrid photocatalyst for the removal of cationic dyes: Mechanistic and degradation pathways. J. Environ. Manag. 2020, 273, 111125. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Yang, H.; Wang, H.; Li, H.; Wu, S.; Yang, W. PMS activation by magnetic cobalt-N-doped carbon composite for ultra-efficient degradation of refractory organic pollutant: Mechanisms and identification of intermediates. Chemosphere 2022, 287, 132074. [Google Scholar] [CrossRef]
- Pang, Y.; Kong, L.; Chen, D.; Yuvaraja, G.; Mehmood, S. Facilely synthesized cobalt doped hydroxyapatite as hydroxyl promoted peroxymonosulfate activator for degradation of Rhodamine B. J. Hazard. Mater. 2020, 384, 121447. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Kim, W.; Alfarraj, S.; Alharbi, S.A. Facile fabrication of (2D/2D) MoS2@MIL-88(Fe) interface-driven catalyst for efficient degradation of organic pollutants under visible light irradiation. J. Hazard. Mater. 2021, 414, 125522. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Zhang, D.; Lan, Y.; Guo, J. Enhanced kinetic performance of peroxymonosulfate/ZVI system with the addition of copper ions: Reactivity, mechanism, and degradation pathways. J. Hazard. Mater. 2020, 393, 121209. [Google Scholar] [CrossRef]
- Chen, S.; Ma, L.; Du, Y.; Zhan, W.; Zhang, T.C.; Du, D. Highly efficient degradation of rhodamine B by carbon nanotubes-activated persulfate. Sep. Purif. Technol. 2021, 256, 117788. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, E.; Xu, D.; Guo, Q. Activation of peroxymonosulfate by bimetallic CoMn oxides loaded on coal fly ash-derived SBA-15 for efficient degradation of Rhodamine B. Sep. Purif. Technol. 2021, 274, 119081. [Google Scholar] [CrossRef]
- Hu, L.; Deng, G.; Lu, W.; Lu, Y.; Zhang, Y. Peroxymonosulfate activation by Mn3O4/metal-organic framework for degradation of refractory aqueous organic pollutant rhodamine B. Chin. J. Catal. 2017, 38, 1360–1372. [Google Scholar] [CrossRef]
- Liang, L.; Cheng, L.; Zhang, Y.; Wang, Q.; Meng, X. Efficiency and mechanisms of rhodamine B degradation in Fenton-like systems based on zero-valent iron. RSC Adv. 2020, 10, 28509–28515. [Google Scholar] [CrossRef]
- Kong, L.; Fang, G.; Chen, Y.; Xie, M.; Zhu, F.; Ma, L.; Zhou, D.; Zhan, J. Efficient activation of persulfate decomposition by Cu2FeSnS4 nanomaterial for bisphenol A degradation: Kinetics, performance and mechanism studies. Appl. Catal. B Environ. 2019, 253, 278–285. [Google Scholar] [CrossRef]


















| Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | |
|---|---|---|---|
| CM | 35.45 | 0.1803 | 20.34 |
| CM/g-C3N4 | 42.72 | 0.2092 | 19.59 |
| C/g-C3N4 | 42.18 | 0.1992 | 18.89 |
| M/g-C3N4 | 58.47 | 0.2041 | 13.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, Y.; Xu, W.; Zhang, X.; Zhou, S. Enhanced Degradation of Rhodamine B through Peroxymonosulfate Activated by a Metal Oxide/Carbon Nitride Composite. Water 2022, 14, 2054. https://doi.org/10.3390/w14132054
Mo Y, Xu W, Zhang X, Zhou S. Enhanced Degradation of Rhodamine B through Peroxymonosulfate Activated by a Metal Oxide/Carbon Nitride Composite. Water. 2022; 14(13):2054. https://doi.org/10.3390/w14132054
Chicago/Turabian StyleMo, Yuanmin, Wei Xu, Xiaoping Zhang, and Shaoqi Zhou. 2022. "Enhanced Degradation of Rhodamine B through Peroxymonosulfate Activated by a Metal Oxide/Carbon Nitride Composite" Water 14, no. 13: 2054. https://doi.org/10.3390/w14132054
APA StyleMo, Y., Xu, W., Zhang, X., & Zhou, S. (2022). Enhanced Degradation of Rhodamine B through Peroxymonosulfate Activated by a Metal Oxide/Carbon Nitride Composite. Water, 14(13), 2054. https://doi.org/10.3390/w14132054