CO2 Addition and Semicontinuous Feed Regime in Shaded HRAP—Pathogen Removal Performance
Abstract
:1. Introduction
2. Materials and Methods
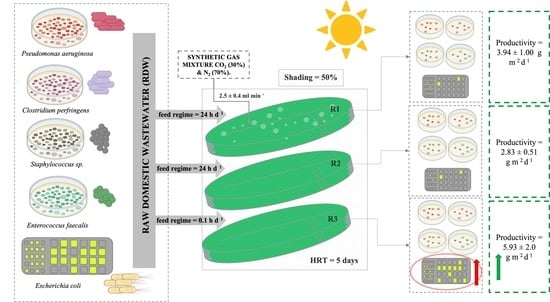
2.1. Experimental Setup and Operation Conditions
2.2. Raw Sewage, Microorganisms, and Culture Conditions
2.3. Continuous Experiment and Sampling
2.4. Analytical Procedures
2.5. Statistical
3. Results and Discussion
3.1. Operation and Environmental Conditions
3.2. Influence of Solar Radiation and CO2 Addition on Pathogen Removal Efficiency
3.2.1. Pseudomonas Aeruginosa
3.2.2. Clostridium Perfringens
3.2.3. Staphylococcus sp.
3.2.4. Enterococcus sp.
3.2.5. Escherichia coli
3.3. Organic Matter, Carbon, and Nutrient Removal Efficiencies
3.4. Productivity and Settleability of Biomass and Microalgae Population
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for Antibiotic Resistant Bacteria and Genes Spread into the Environment: A Review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Nicco, E.; Mikulska, M. Why Is Community-Associated MRSA Spreading across the World and How Will It Change Clinical Practice? Int. J. Antimicrob. Agents 2009, 34, S15–S19. [Google Scholar] [CrossRef]
- Gómez-López, M.D.; Bayo, J.; García-Cascales, M.S.; Angosto, J.M. Decision Support in Disinfection Technologies for Treated Wastewater Reuse. J. Clean. Prod. 2009, 17, 1504–1511. [Google Scholar] [CrossRef]
- Gehr, R.; Wagner, M.; Veerasubramanian, P.; Payment, P. Disinfection Efficiency of Peracetic Acid, UV and Ozone after Enhanced Primary Treatment of Municipal Wastewater. Water Res. 2003, 37, 4573–4586. [Google Scholar] [CrossRef]
- Saúco, C.; Cano, R.; Marín, D.; Lara, E.; Rogalla, F.; Arbib, Z. Hybrid Wastewater Treatment System Based in a Combination of High Rate Algae Pond and Vertical Constructed Wetland System at Large Scale. J. Water Process Eng. 2021, 43, 102311. [Google Scholar] [CrossRef]
- Ruas, G.; Farias, S.L.; Scarcelli, P.G.; Serejo, M.L.; Boncz, M.A. The Effect of CO2 Addition and Hydraulic Retention Time on Pathogens Removal in HRAPs. Water Sci. Technol. 2020, 82, 1184–1192. [Google Scholar] [CrossRef]
- Posadas, E.; del Morales, M.M.; Gomez, C.; Acién, F.G.; Muñoz, R. Influence of PH and CO2 Source on the Performance of Microalgae-Based Secondary Domestic Wastewater Treatment in Outdoors Pilot Raceways. Chem. Eng. J. 2015, 265, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Vassalle, L.; García-Galán, M.J.; Aquino, S.F.; de Afonso, R.J.C.F.; Ferrer, I.; Passos, F.; R Mota, C. Can High Rate Algal Ponds Be Used as Post-Treatment of UASB Reactors to Remove Micropollutants? Chemosphere 2020, 248, 125969. [Google Scholar] [CrossRef]
- Saavedra, R.; Muñoz, R.; Taboada, M.E.; Bolado, S. Influence of Organic Matter and CO2 Supply on Bioremediation of Heavy Metals by Chlorella Vulgaris and Scenedesmus Almeriensis in a Multimetallic Matrix. Ecotoxicol. Environ. Saf. 2019, 182, 109393. [Google Scholar] [CrossRef] [Green Version]
- Ruas, G.; Serejo, M.L.; Paulo, P.L.; Boncz, M.Á. Evaluation of Domestic Wastewater Treatment Using Microalgal-Bacterial Processes: Effect of CO2 Addition on Pathogen Removal. J. Appl. Phycol. 2018, 30, 921–929. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talaiekhozani, A.; Rezania, S. Application of Photosynthetic Bacteria for Removal of Heavy Metals, Macro-Pollutants and Dye from Wastewater: A Review. J. Water Process Eng. 2017, 19, 312–321. [Google Scholar] [CrossRef]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic Digestion of Algae Biomass: A Review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Craggs, R.J.; Park, J.; Sukias, J.P.S.; Nagels, J.W.; Stott, R. Virus Removal in a Pilot-Scale “advanced” Pond System as Indicated by Somatic and F-RNA Bacteriophages. Water Sci. Technol. 2005, 51, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.P.; Mara, D.D.; Silva, S.A. Influence of PH, Oxygen, and Humic Substances on Ability of Sunlight to Dammage Fecal-Coliforms in Waste Stabilisation Pond Water. Appl. Environ. Microbiol. 1992, 58, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Assemany, P.P.; Calijuri, M.L.; Couto, E.d.A.d.; de Souza, M.H.B.; Silva, N.C.; da Santiago, A.F.; de Castro, J.S. Algae/Bacteria Consortium in High Rate Ponds: Influence of Solar Radiation on the Phytoplankton Community. Ecol. Eng. 2015, 77, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Assemany, P.P.; Calijuri, M.L.; da Santiago, A.F.; Couto, E.d.A.d.; de Leite, M.O.; Bermudez Sierra, J.J. Effect of Solar Radiation on the Lipid Characterization of Biomass Cultivated in High-Rate Algal Ponds Using Domestic Sewage. Environ. Technol. 2014, 35, 2296–2305. [Google Scholar] [CrossRef]
- Serejo, M.L.; Posadas, E.; Boncz, M.A.; Blanco, S.; García-Encina, P.; Muñoz, R. Influence of Biogas Flow Rate on Biomass Composition during the Optimization of Biogas Upgrading in Microalgal-Bacterial Processes. Environ. Sci. Technol. 2015, 49, 3228–3236. [Google Scholar] [CrossRef]
- Sournia, A. Phytoplankton Manual; United Nations Educational, Scientific and Cultural Organization, Eds.; Muséum National d’Histoire Naturelle: Paris, France, 1978; ISBN 92-3-101572-9. [Google Scholar]
- APHA. Standard Methods for the Examination of Water, 22nd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- Zaiontz, C. The Data Analysis for This Paper Was Generated Using the Real Statistics Resource Pack Software (Release 7.6). 2020. Available online: www.real-statistics.com (accessed on 24 October 2022).
- Guieysse, B.; Béchet, Q.; Shilton, A. Variability and Uncertainty in Water Demand and Water Footprint Assessments of Fresh Algae Cultivation Based on Case Studies from Five Climatic Regions. Bioresour. Technol. 2013, 128, 317–323. [Google Scholar] [CrossRef]
- Serejo, M.L.; Farias, S.L.; Ruas, G.; Paulo, P.L.; Boncz, M.A. Surfactant Removal and Biomass Production in a Microalgal-Bacterial Process: Effect of Feeding Regime. Water Sci. Technol. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Posadas, E.; Muñoz, A.; García-González, M.-C.; Muñoz, R.; García-Encina, P.A. A Case Study of a Pilot High Rate Algal Pond for the Treatment of Fish Farm and Domestic Wastewaters. J. Chem. Technol. Biotechnol. 2014, 90, 1094–1101. [Google Scholar] [CrossRef]
- Metcalf & Eddy, I.; Burton, F.L.; Tchobanoglous, G.; Tsuchihashi, R.; Stensel, H.D. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill’s Education: New York, NY, USA, 2013. [Google Scholar]
- Syed, S.; Arasu, A.; Ponnuswamy, I. The Uses of Chlorella Vulgaris as Antimicrobial Agent and as a Diet: The Presence of Bio-Active Compounds Which Caters the Vitamins, Minerals in General. Int. J. Bio-Sci. Bio-Technol. 2015, 7, 185–190. [Google Scholar] [CrossRef]
- Maghembe, R.; Damian, D.; Makaranga, A.; Nyandoro, S.S.; Lyantagaye, S.L.; Kusari, S.; Hatti-Kaul, R. Omics for Bioprospecting and Drug Discovery from Bacteria and Microalgae. Antibiotics 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G. Wastewater Microbiology, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 53, ISBN 978-0-471-71791-1. [Google Scholar]
- Payment, P.; Franco, E. Clostridium Perfringens and Somatic Coliphages as Indicators of the Efficiency of Drinking Water Treatment for Viruses and Protozoan Cysts. Appl. Environ. Microbiol. 1993, 59, 2418–2424. [Google Scholar] [CrossRef] [Green Version]
- Venczel, L.V.; Arrowood, M.; Hurd, M.; Sobsey, M. Inactation of Cryptosporidium Parvum Oocyts and Clostridium Perfringens Spores by a Mixed-Oxidant Disinfectant and by Free Chlorine. Appl. Environ. Microbiol. 1997, 63, 1598–1601. [Google Scholar] [CrossRef] [Green Version]
- European Union. Directiva 98/15/CE de La Comisión de 27 de Febrero de 1998 Por La Que Se Modifica La Directiva 91/271/CEE Del Consejo En Relación Con Determinados Requisitos Establecidos En Su Anexo I; European Union: Maastricht, The Netherlands, 1998; pp. L67/29–L67/30. [Google Scholar]
- García, M.; Soto, F.; González, J.M.; Bécares, E. A Comparison of Bacterial Removal Efficiencies in Constructed Wetlands and Algae-Based Systems. Ecol. Eng. 2008, 32, 238–243. [Google Scholar] [CrossRef]
- Lanao, M.; Ormad, M.P.; Goñi, P.; Miguel, N.; Mosteo, R.; Ovelleiro, J.L. Inactivation of Clostridium Perfringens Spores and Vegetative Cells by Photolysis and TiO2 Photocatalysis with H2O2. Sol. Energy 2010, 84, 703–709. [Google Scholar] [CrossRef]
- Gutiérrez-alfaro, S.; Rueda-márquez, J.J.; Perales, J.A.; Manzano, M.A. Combining Sun-Based Technologies (Microalgae and Solar Disinfection) for Urban Wastewater Regeneration. Sci. Total Environ. 2018, 619–620, 1049–1057. [Google Scholar] [CrossRef]
- Bailey, E.S.; Casanova, L.M.; Sobsey, M.D. Effects of Environmental Storage Conditions on Survival of Indicator Organisms in a Blend of Surface Water and Dual Disinfected Reclaimed Water. J. Appl. Microbiol. 2019, 126, 985–994. [Google Scholar] [CrossRef]
- Krustok, I.; Truu, J.; Odlare, M.; Truu, M.; Ligi, T.; Tiirik, K.; Nehrenheim, E. Effect of Lake Water on Algal Biomass and Microbial Community Structure in Municipal Wastewater-Based Lab-Scale Photobioreactors. Appl. Microbiol. Biotechnol. 2015, 99, 6537–6549. [Google Scholar] [CrossRef]
- Nola, M.; Matchim, A.G.S.; Mobili, O.B.; Nougang, M.; Krier, F.; Chihib, N.E.; Hornez, J.P.; Njiné, T. Photoinactivation of Staphylococcus Aureus and Vibrio Parahaemolyticus in the Model Aquatic Microcosm: Effect of Light Intensity and Dissolved Biodegradable Organic Compound. Water Sci. Technol. 2010, 62, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Saavedra, M.D.P.; Licea-Navarro, A.; Bernáldez-Sarabia, J. Evaluation of the Antibacterial Activity of Different Species of Phytoplankton. Rev. Biol. Mar. Oceanogr. 2010, 45, 531–536. [Google Scholar] [CrossRef]
- Awuah, E.; Lubberding, H.J.; Asante, K.; Gijzen, H.J. The Effect of PH on Enterococci Removal in Pistia-, Duckweed- and Algae-Based Stabilization Ponds for Domestic Wastewater Treatment. Water Sci. Technol. 2002, 45, 67–74. [Google Scholar] [CrossRef]
- Davies-Colley, R.; Donnison, A.M.; Speed, D.J. Sunlight Wavelengths Inactivating Faecal Indicator Microorganisms in Waste Stabilisation Ponds. Water Sci. Technol. 1997, 35, 219–225. [Google Scholar] [CrossRef]
- Wu, S.Y.; Klein, D.A. Starvation Effects on Escherichia Coli and Aquatic Bacterial Responses to Nutrient Addition and Secondary Warming Stresses. Appl. Environ. Microbiol. 1976, 31, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Legendre, P.; Baleux, B.; Troussellier, M. Dynamics of Pollution-Indicator and Heterotrophic Bacteria in Sewage Treatment Lagoons. Appl. Environ. Microbiol. 1984, 48, 586–593. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; Regional Office for the Eastern Mediterranean. A Compendium of Drinking-Water Quality Standards in the Eastern Mediterranean Region; WHO: Geneve, Switzerland, 2006; p. 24. Available online: https://apps.who.int/iris/handle/10665/116514 (accessed on 24 October 2022).
- Ouali, A.; Jupsin, H.; Ghrabi, A.; Vasel, J.L. Removal Kinetic of Escherichia Coli and Enterococci in a Laboratory Pilot Scale Wastewater Maturation Pond. Water Sci. Technol. 2014, 69, 755–759. [Google Scholar] [CrossRef]
- Craggs, R.; Park, J.; Heubeck, S.; Sutherland, D. High Rate Algal Pond Systems for Low-Energy Wastewater Treatment, Nutrient Recovery and Energy Production. N. Z. J. Bot. 2014, 52, 60–73. [Google Scholar] [CrossRef]
- Kim, B.H.; Kang, Z.; Ramanan, R.; Choi, J.E.; Cho, D.H.; Oh, H.M.; Kim, H.S. Nutrient Removal and Biofuel Production in High Rate Algal Pond Using Real Municipal Wastewater. J. Microbiol. Biotechnol. 2014, 24, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A.; Borowitzka, L.J. Micro-Algal Biotechnology; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Posadas, E.; García-Encina, P.-A.; Soltau, A.; Domínguez, A.; Díaz, I.; Muñoz, R. Carbon and Nutrient Removal from Centrates and Domestic Wastewater Using Algal-Bacterial Biofilm Bioreactors. Bioresour. Technol. 2013, 139, 50–58. [Google Scholar] [CrossRef]
- Hoffmann, J.P. Minireview Wastewater Treatment with Suspended and Nonsuspended Algae 1. J. Phycol. 1998, 763, 757–763. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Recycling Algae to Improve Species Control and Harvest Efficiency from a High Rate Algal Pond. Water Res. 2011, 45, 6637–6649. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.K.; Craggs, R.J. Wastewater Treatment and Algal Production in High Rate Algal Ponds with Carbon Dioxide Addition. Water Sci. Technol. 2010, 61, 633–639. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Unit | RS |
|---|---|---|
| COD | mg COD L−1 | 127.45 ± 48.43 |
| TOC | mg C L−1 | 127.50 ± 10.61 |
| IC | mg C L−1 | 60.00 ± 5.66 |
| TN | mg N L−1 | 45.00 ± 35.49 |
| NH4+ | mg N-NH4+ L−1 | 21.43 ± 15.25 |
| NO2− | mg N-NO2−L−1 | ND |
| NO3− | mg N-NO3−L−1 | ND |
| P | mg P-PO43− L−1 | 6.09 ± 0.45 |
| C:N | - | 2.8 |
| C/N/P | - | 1 July 2022 |
| pH | - | 7.78 ± 0.18 |
| Pseudomonas aeruginosa | CFU (100 mL)−1 | (3.46 ± 5.99) × 105 |
| Clostridium perfringens | CFU (100 mL)−1 | (3.46 ± 5.99) × 105 |
| Staphylococcus sp. | CFU (100 mL)−1 | (3.46 ± 5.99) × 105 |
| Enterococcus faecalis | CFU (100 mL)−1 | (3.46 ± 5.99) × 105 |
| Escherichia coli | MPN (100 mL)−1 | (3.46 ± 5.99) × 105 |
| Parameters | Unit | R1 | R2 | R3 |
|---|---|---|---|---|
| HRT | d | 4.97 ± 0.34 | 4.88 ± 0.33 | 5.00 ± 0.00 |
| OLR | g COD m2 d−1 | 4.08 ± 0.30 | 4.07 ± 0.28 | 3.94 ± 0.00 |
| Shading | % | 50 | 50 | 50 |
| CO2 addition | mL min−1 | 2.5 ± 0.4 | - | - |
| Fed regime | h | 24 | 24 | 0.1 |
| pH | 7.27 ± 0.79 | 7.46 ± 0.57 | 7.38 ± 1.07 | |
| DO | mg O2 L−1 | 7.30 ± 1.07 | 7.12 ± 1.13 | 6.83 ± 1.22 |
| Light Intensity ** | μmol m−2 s−1 | 507.2 ± 278.2 | ||
| Temperature | °C | 21.59 ± 3.56 | 21.65 ± 3.57 | 21.73 ± 3.46 |
| Evaporation rate | L m−2 d−1 | 6.79 ± 7.08 | 5.21 ± 7.51 | 6.04 ± 6.85 |
| Productivity | g m−2 d−1 | 3.94 ± 1.00 * | 2.83 ± 0.51 * | 5.93 ± 2.0 * |
| TSS | g L−1 | 0.18 ± 0.04 | 0.13 ± 0.02 | 0.29 ± 0.09 * |
| Sed-Re | % | 31.51 ± 25.48 | −52.38 ± 104.79 * | 36.49 ± 27.26 |
| Removal Efficiency (%) | ||||||
|---|---|---|---|---|---|---|
| HRAP | COD-Re | TOC-Re | IC-Re | TC-Re | TN-Re | TP-Re |
| R1 | 63.26 ± 8.21 | 31.02 ± 31.94 | 67.36 ± 10.64 | 42.62 ± 25.21 | 73.40 ± 3.15 | 28.98 ± 7.29 |
| R2 | 65.65 ± 12.59 | 38.76 ± 17.41 | 69.79 ± 10.44 | 48.76 ± 14.72 | 67.28 ± 21.47 | 26.57 ± 11.92 |
| R2 | 59.36 ± 15.68 | 36.94 ± 24.19 | 66.23 ± 11.95 | 46.44 ± 20.54 | 59.67 ± 18.31 | 35.05 ± 14.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruas, G.; Lacerda, S.F.; Nantes, M.A.; Serejo, M.L.; da Silva, G.H.R.; Boncz, M.Á. CO2 Addition and Semicontinuous Feed Regime in Shaded HRAP—Pathogen Removal Performance. Water 2022, 14, 4047. https://doi.org/10.3390/w14244047
Ruas G, Lacerda SF, Nantes MA, Serejo ML, da Silva GHR, Boncz MÁ. CO2 Addition and Semicontinuous Feed Regime in Shaded HRAP—Pathogen Removal Performance. Water. 2022; 14(24):4047. https://doi.org/10.3390/w14244047
Chicago/Turabian StyleRuas, Graziele, Sarah Farias Lacerda, Maria Alice Nantes, Mayara Leite Serejo, Gustavo Henrique Ribeiro da Silva, and Marc Árpad Boncz. 2022. "CO2 Addition and Semicontinuous Feed Regime in Shaded HRAP—Pathogen Removal Performance" Water 14, no. 24: 4047. https://doi.org/10.3390/w14244047
APA StyleRuas, G., Lacerda, S. F., Nantes, M. A., Serejo, M. L., da Silva, G. H. R., & Boncz, M. Á. (2022). CO2 Addition and Semicontinuous Feed Regime in Shaded HRAP—Pathogen Removal Performance. Water, 14(24), 4047. https://doi.org/10.3390/w14244047