Preparation of Magnetic Dummy Molecularly Imprinted Meso-Porous Silica Nanoparticles Using a Semi-Covalent Imprinting Approach for the Rapid and Selective Removal of Bisphenols from Environmental Water Samples
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Apparatus
2.2. Characterisation of the m-DMI-MSNPs and m-NI-MSNPs
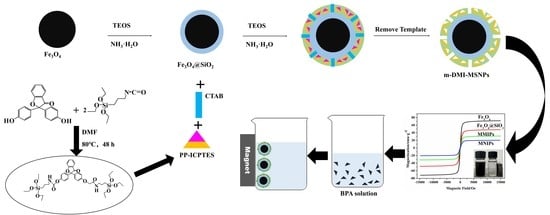
2.3. Preparation of the m-DMI-MSNPs and m-NI-MSNPs
2.3.1. Preparation of Magnetic Fe3O4
2.3.2. Preparation of Fe3O4@SiO2
2.3.3. Preparation of the Template–Monomer Complexes
2.3.4. Preparation of m-DMI-MSNPs and m-NI-MSNPs
2.4. Adsorption Experiments of the m-DMI-MSNPs and m-NI-MSNPs
2.4.1. Static Adsorption
2.4.2. Competition Studies
2.4.3. Adsorption Equilibrium Time
2.5. Removal of BPs from the Aqueous Solution
2.6. HPLC Detection
3. Results and Discussion
3.1. Selection of the Dummy Template
Morphology and Characterization
3.2. Binding Properties
3.2.1. Adsorption Isotherm
3.2.2. Adsorption Equilibrium Time
3.2.3. Adsorption Selectivity
3.3. Application of m-DMI-MSNPs in Water Samples
3.4. Comparison of the MMIP Properties for BPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lin, Z.; Cheng, W.; Li, Y.; Liu, Z.; Chen, X.; Huang, C. A Novel Superparamagnetic Surface Molecularly Imprinted Nanoparticle Adopting Dummy Template: An Efficient Solid-Phase Extraction Adsorbent for Bisphenol A. Anal. Chim. Acta 2012, 720, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wu, L.-H.; Liu, G.-Q.; Shi, L.; Guo, Y. Occurrence and Ecological Risk Assessment of Eight Endocrine-Disrupting Chemicals in Urban River Water and Sediments of South China. Arch. Environ. Contam. Toxicol. 2018, 75, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Bi, X.; Peng, Y.; Bai, M. Research Advances of the Phosphorus-Accumulating Organisms of Candidatus Accumulibacter, Dechloromonas and Tetrasphaera: Metabolic Mechanisms, Applications and Influencing Factors. Chemosphere 2022, 307, 135675. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Endocrine Disrupting Chemicals and Breast Cancer: A Systematic Review of Epidemiological Studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 6549–6576. [Google Scholar] [CrossRef]
- den Braver-Sewradj, S.P.; van Spronsen, R.; Hessel, E.V.S. Substitution of Bisphenol A: A Review of the Carcinogenicity, Reproductive Toxicity, and Endocrine Disruption Potential of Alternative Substances. Crit. Rev. Toxicol. 2020, 50, 128–147. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Z.; Liu, Z.; Zeng, F.; Zhang, J.; Dang, Z. Occurrence, Spatial Distribution, and Main Source Identification of Ten Bisphenol Analogues in the Dry Season of the Pearl River, South China. Environ. Sci. Pollut. Res. 2022, 29, 27352–27365. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Tang, Z.; Zhang, J.; Yin, H.; Dang, Z.; Wu, P.; Liu, Y. Bisphenol Analogues in Chinese Bottled Water: Quantification and Potential Risk Analysis. Sci. Total Environ. 2020, 713, 136583. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Yu, K.; Li, L.; Zhang, Z.; Li, L. A Versatile Strategy to Fabricate Magnetic Dummy Molecularly Imprinted Mesoporous Silica Particles for Specific Magnetic Separation of Bisphenol A. New J. Chem. 2019, 43, 3400–3408. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Du, F.; Zheng, W.; Liu, Z.; Xu, Z.; Hu, X.; Liu, H. Dummy-Template Molecularly Imprinted Micro-Solid-Phase Extraction Coupled with High-Performance Liquid Chromatography for Bisphenol A Determination in Environmental Water Samples. Microchem. J. 2019, 145, 337–344. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Wu, W.; Wang, Z.; Chu, Y.; Chen, X. Hollow Porous Dummy Molecularly Imprinted Polymer as a Sorbent of Solid-Phase Extraction Combined with Accelerated Solvent Extraction for Determination of Eight Bisphenols in Plastic Products. Microchem. J. 2019, 145, 1176–1184. [Google Scholar] [CrossRef]
- Chang, T.; Yan, X.; Liu, S.; Liu, Y. Magnetic Dummy Template Silica Sol–Gel Molecularly Imprinted Polymer Nanospheres as Magnetic Solid-Phase Extraction Material for the Selective and Sensitive Determination of Bisphenol A in Plastic Bottled Beverages. Food Anal. Methods 2017, 10, 3980–3990. [Google Scholar] [CrossRef]
- Kubiak, A.; Ciric, A.; Biesaga, M. Dummy Molecularly Imprinted Polymer (DMIP) as a Sorbent for Bisphenol S and Bisphenol F Extraction from Food Samples. Microchem. J. 2020, 156, 104836. [Google Scholar] [CrossRef]
- Lee, J.; Moon, K.W.; Ji, K. Systematic Review of Exposure to Bisphenol A Alternatives and Its Effects on Reproduction and Thyroid Endocrine System in Zebrafish. Appl. Sci. 2021, 11, 1837. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Li, Y.; Jin, J.; Yang, J.; Li, F.; Shah, S.M.; Chen, J. Highly Class-Selective Solid-Phase Extraction of Bisphenols in Milk, Sediment and Human Urine Samples Using Well-Designed Dummy Molecularly Imprinted Polymers. J. Chromatogr. A 2014, 1360, 9–16. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Huang, C.; Jiao, Y.; Chen, J. A Phenolphthalein-Dummy Template Molecularly Imprinted Polymer for Highly Selective Extraction and Clean-Up of Bisphenol A in Complex Biological, Environmental and Food Samples. Polymers 2018, 10, 1150. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Li, D.; Du, Z.; Xiong, C.; Jiang, H. Magnetic Solid-Phase Extraction Modified Quick, Easy, Cheap, Effective, Rugged and Safe Method Combined with Pre-Column Derivatization and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry for Determination of Estrogens and Estrogen Mimics in Pork and Chicken Samples. J. Chromatogr. A 2020, 1622, 461137. [Google Scholar] [CrossRef]
- Cheng, Y.; Nie, J.; Liu, H.; Kuang, L.; Xu, G. Synthesis and Characterization of Magnetic Molecularly Imprinted Polymers for Effective Extraction and Determination of Kaempferol from Apple Samples. J. Chromatogr. A 2020, 1630, 461531. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Lu, W.; Wang, X.; Li, J.; You, H.; Xiong, H.; Chen, L. Water-Compatible Temperature and Magnetic Dual-Responsive Molecularly Imprinted Polymers for Recognition and Extraction of Bisphenol A. J. Chromatogr. A 2016, 1435, 30–38. [Google Scholar] [CrossRef]
- Liu, Y.; Song, W.; Zhou, D.; Han, F.; Gong, X.; Pan, P. A New Core–Shell Magnetic Mesoporous Surface Molecularly Imprinted Composite and Its Application as an MSPE Sorbent for Determination of Phthalate Esters. RSC Adv. 2022, 12, 7253–7261. [Google Scholar] [CrossRef]
- Wu, Q.; Li, M.; Huang, Z.; Shao, Y.; Bai, L.; Zhou, L. Well-Defined Nanostructured Core–Shell Magnetic Surface Imprinted Polymers (Fe 3 O 4 @SiO 2 @MIPs) for Effective Extraction of Trace Tetrabromobisphenol A from Water. J. Ind. Eng. Chem. 2018, 60, 268–278. [Google Scholar] [CrossRef]
- Bing-zhi, D.; Hua-qiang, C.; Lin, W.; Sheng-ji, X.; Nai-yun, G. The Removal of Bisphenol A by Hollow Fiber Microfiltration Membrane. Desalination 2010, 250, 693–697. [Google Scholar] [CrossRef]
- Huang, D.; Tang, Z.; Peng, Z.; Lai, C.; Zeng, G.; Zhang, C.; Xu, P.; Cheng, M.; Wan, J.; Wang, R. Fabrication of Water-Compatible Molecularly Imprinted Polymer Based on β-Cyclodextrin Modified Magnetic Chitosan and Its Application for Selective Removal of Bisphenol A from Aqueous Solution. J. Taiwan Inst. Chem. Eng. 2017, 77, 113–121. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Chu, J.; Dong, C.; Qi, J.; Yuan, Y. Synthesis of Core-Shell Magnetic Molecular Imprinted Polymer by the Surface RAFT Polymerization for the Fast and Selective Removal of Endocrine Disrupting Chemicals from Aqueous Solutions. Environ. Pollut. 2010, 158, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yin, J.; Xu, Z.; Zhao, C.; Huang, H.; Zhang, H.; Wang, C. Preparation of Magnetic Molecularly Imprinted Polymer for Rapid Determination of Bisphenol A in Environmental Water and Milk Samples. Anal Bioanal. Chem. 2009, 395, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Karrat, A.; Amine, A. Solid-Phase Extraction Combined with a Spectrophotometric Method for Determination of Bisphenol-A in Water Samples Using Magnetic Molecularly Imprinted Polymer. Microchem. J. 2021, 168, 106496. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, L.; Mao, X.; Tan, T.; Wan, H.; Wan, Y. A Magnetic Hydrophilic Molecularly Imprinted Material with Multiple Stimuli-Response Properties for Efficient Recognition of Bisphenol A in Beverages. Food Chem. 2020, 331, 127311. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Chen, W.; Li, Y.; Wei, Y.; Luo, A. Magnetic Fluorescence Molecularly Imprinted Polymer Based on FeOx/ZnS Nanocomposites for Highly Selective Sensing of Bisphenol A. Polymers 2019, 11, 1210. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Liu, Y.; Teng, W.; Tan, J.; Liang, Y.; Tang, Y. Preparation of Core-Shell Magnetic Molecular Imprinted Polymer with Binary Monomer for the Fast and Selective Extraction of Bisphenol A from Milk. J. Chromatogr. A 2016, 1462, 2–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Rao, W.; Chen, H.; Cai, R. Synthesis and Properties of Magnetic Molecularly Imprinted Polymers Based on Multiwalled Carbon Nanotubes for Magnetic Extraction of Bisphenol A from Water. J. Chromatogr. B 2014, 965, 190–196. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Cao, Y.-H.; Cao, G.-Q. Preparation and Application of Core-Shell Magnetic Imprinted Nanoparticles for Bisphenol A. Chin. J. Anal. Chem. 2013, 41, 1724–1728. [Google Scholar] [CrossRef]
- Rao, W.; Cai, R.; Yin, Y.; Long, F.; Zhang, Z. Magnetic Dummy Molecularly Imprinted Polymers Based on Multi-Walled Carbon Nanotubes for Rapid Selective Solid-Phase Extraction of 4-Nonylphenol in Aqueous Samples. Talanta 2014, 128, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Su, Y.; Qi, J.; Jia, Y.; Huang, C.; Dong, Q. Selective Extraction of Bisphenol A from Water by One-Monomer Molecularly Imprinted Magnetic Nanoparticles. J. Sep. Sci. 2018, 41, 2029–2036. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Zhu, X.; He, C.; Wang, Q.; Liu, S. Dummy Molecularly Imprinted Magnetic Nanoparticles for Dispersive Solid-Phase Extraction and Determination of Bisphenol A in Water Samples and Orange Juice. Talanta 2017, 162, 57–64. [Google Scholar] [CrossRef] [PubMed]






| Tap Water | Mineral Water | Sewage Influents | ||||
|---|---|---|---|---|---|---|
| ± SD (%) | RSD (n = 3) | ± SD (%) | RSD (n = 3) | ± SD (%) | RSD (n = 3) | |
| BPF | 96.62 ± 2.71 | 2.8% | 95.55 ± 1.44 | 1.5% | 93.07 ± 6.33 | 6.8% |
| BPE | 97.84 ± 2.25 | 2.3% | 97.13 ± 1.36 | 1.4% | 94.29 ± 1.32 | 1.4% |
| BPA | 97.08 ± 3.20 | 3.3% | 96.73 ± 2.81 | 2.9% | 95.33 ± 2.19 | 2.3% |
| Template | α | Qmax (mg g−1) | Saturation Magnetization (emu g−1) | Equilibrium Time (min) | Reference |
|---|---|---|---|---|---|
| BPA | 4.1 | 21.30 | 0.14 | 20 | [23] |
| BPA | 1.4 | 0.39 | 60 | 5 | [24] |
| BPA | 1.71 | 60 | - | 10 | [25] |
| BPA | 4.25 | 8.97 | 29.01 | 240 | [26] |
| BPA | 11.19 | 50.92 | 24.58 | 5 | [27] |
| BPA | 1.10 | 105.5 | <6 | 60 | [22] |
| BPA | 3.87 | 17.98 | 35.18 | 40 | [28] |
| BPA | 3.95 | 8.29 | 38.3 | 500 | [18] |
| BPA | 3.29 | 11.00 | 41.1 | 15 | [29] |
| BPA | 3.5 | 122.2 | 28.01 | 120 | [30] |
| PTOP | 1.8 | 10.64 | 26.52 | 20 | [31] |
| BPF | 1.3 | 26.53 | 30.1 | 5 | [32] |
| BPAF | - | 5.92 | 37.75 | 2 | [33] |
| 4,4’-Biphenol | 4.8 | 76.80 | 4.87 | 180 | [8] |
| DDBP | 2.04 | 101.49 | 47.60 | 30 | [11] |
| PP | 7.5 | 38.75 | 30.68 | <1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Sun, X.; Wang, M.; Wang, Y.; Wu, Q.; Wu, S.; Fang, S. Preparation of Magnetic Dummy Molecularly Imprinted Meso-Porous Silica Nanoparticles Using a Semi-Covalent Imprinting Approach for the Rapid and Selective Removal of Bisphenols from Environmental Water Samples. Water 2022, 14, 4125. https://doi.org/10.3390/w14244125
Chen J, Sun X, Wang M, Wang Y, Wu Q, Wu S, Fang S. Preparation of Magnetic Dummy Molecularly Imprinted Meso-Porous Silica Nanoparticles Using a Semi-Covalent Imprinting Approach for the Rapid and Selective Removal of Bisphenols from Environmental Water Samples. Water. 2022; 14(24):4125. https://doi.org/10.3390/w14244125
Chicago/Turabian StyleChen, Jing, Xiaoli Sun, Muhua Wang, Yan Wang, Qinyao Wu, Shurong Wu, and Sisi Fang. 2022. "Preparation of Magnetic Dummy Molecularly Imprinted Meso-Porous Silica Nanoparticles Using a Semi-Covalent Imprinting Approach for the Rapid and Selective Removal of Bisphenols from Environmental Water Samples" Water 14, no. 24: 4125. https://doi.org/10.3390/w14244125
APA StyleChen, J., Sun, X., Wang, M., Wang, Y., Wu, Q., Wu, S., & Fang, S. (2022). Preparation of Magnetic Dummy Molecularly Imprinted Meso-Porous Silica Nanoparticles Using a Semi-Covalent Imprinting Approach for the Rapid and Selective Removal of Bisphenols from Environmental Water Samples. Water, 14(24), 4125. https://doi.org/10.3390/w14244125