Equilibrium, Kinetic and Thermodynamic Studies for the Adsorption of Metanil Yellow Using Carbonized Pistachio Shell-Magnetic Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
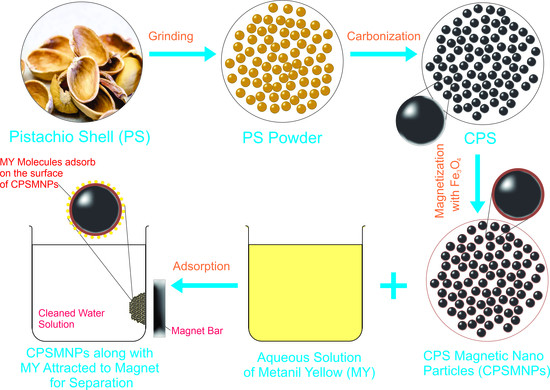
2.2. Preparation of Adsorbent
2.2.1. Synthesis of Carbonized Pistachio Shell (CPS)
2.2.2. Preparation of Carbonized Pistachio Shell Magnetic Nanoparticles (CPSMNPs)
2.3. Characterization
2.4. Application of CPSMNPs for Adsorption
3. Results and Discussion
3.1. Characterization
3.1.1. SEM Analysis
3.1.2. EDX Analysis
3.1.3. BET Analysis
3.2. Effect of Parameters on the Adsorption of MY
3.2.1. Effect of pH
3.2.2. Effect of Contact Time
3.2.3. Effect of Initial Dye Concentration
3.2.4. Effect of Adsorbent Dosage
3.3. Comparison of Adsorption Studies Using PS and CPS with CPSMNPs
3.4. Adsorption Isotherms
3.5. Kinetic Studies
3.6. Thermodynamics Studies
3.7. Comparison of the Current Study with Literature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jan, S.U.; Ahmad, A.; Khan, A.A.; Melhi, S.; Ahmad, I.; Sun, G.; Chen, C.-M.; Ahmad, R. Removal of azo dye from aqueous solution by a low-cost activated carbon prepared from coal: Adsorption kinetics, isotherms study, and DFT simulation. Environ. Sci. Pollut. Res. 2020, 28, 10234–10247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kumar, A.; Naushad, M.; Kumar, A.; Al-Muhtaseb, A.H.; Dhiman, P.; Ghfar, A.A.; Stadler, F.J.; Khan, M. Photoremediation of toxic dye from aqueous environment using monometallic and bimetallic quantum dots-based nanocomposites. J. Clean. Prod. 2018, 172, 2919–2930. [Google Scholar] [CrossRef]
- Garg, D.; Kumar, S.; Sharma, K.; Majumder, C. Application of waste peanut shells to form activated carbon and its utilization for the removal of Acid Yellow 36 from wastewater. Groundw. Sustain. Dev. 2019, 8, 512–519. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.A.; Nazir, M.; Khan, E.A. Adsorptive removal of rhodamine B from textile wastewater using water chestnut (Trapa natans L.) peel: Adsorption dynamics and kinetic studies. Toxicol. Environ. Chem. 2013, 95, 919–931. [Google Scholar] [CrossRef]
- Banerjee, P.; Sau, S.; Das, P.; Mukhopadhayay, A. Optimization and modelling of synthetic azo dye wastewater treatment using Graphene oxide nanoplatelets: Characterization toxicity evaluation and optimization using Artificial Neural Network. Ecotoxicol. Environ. Saf. 2015, 119, 47–57. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F.; Önal, Y. Conversion of grape industrial processing waste to activated carbon sorbent and its performance in cationic and anionic dyes adsorption. J. Clean. Prod. 2015, 93, 84–93. [Google Scholar] [CrossRef]
- Şentürk, I.; Alzein, M. Adsorptive removal of basic blue 41 using pistachio shell adsorbent—Performance in batch and column system. Sustain. Chem. Pharm. 2020, 16, 100254. [Google Scholar] [CrossRef]
- Delnavaz, M.; Mofrad, Z.K. Nano zerovalent iron (NZVI) adsorption performance on acidic dye 36 removal: Optimization of effective factors, isotherm and kinetic study. Environ. Prog. Sustain. Energy 2019, 39, e13349. [Google Scholar] [CrossRef]
- Thirunavukkarasu, A.; Muthukumaran, K.; Nithya, R. Adsorption of acid yellow 36 onto green nanoceria and amine functionalized green nanoceria: Comparative studies on kinetics, isotherm, thermodynamics, and diffusion analysis. J. Taiwan Inst. Chem. Eng. 2018, 93, 211–225. [Google Scholar] [CrossRef]
- Malik, P. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of Acid Yellow 36. Dye. Pigment. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Chiou, M.-S.; Chuang, G.-S. Competitive adsorption of dye metanil yellow and RB15 in acid solutions on chemically cross-linked chitosan beads. Chemosphere 2006, 62, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Ponnusami, V.; Gunasekar, V.; Srivastava, S. Kinetics of methylene blue removal from aqueous solution using gulmohar (Delonix regia) plant leaf powder: Multivariate regression analysis. J. Hazard. Mater. 2009, 169, 119–127. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Arulkumar, M.; Palvannan, T. Utilization of agro-industrial waste Jatropha curcas pods as an activated carbon for the adsorption of reactive dye Remazol Brilliant Blue R (RBBR). J. Clean. Prod. 2012, 22, 67–75. [Google Scholar] [CrossRef]
- Mittal, A.; Gupta, V.; Malviya, A.; Mittal, J. Process development for the batch and bulk removal and recovery of a hazardous, water-soluble azo dye (Metanil Yellow) by adsorption over waste materials (Bottom Ash and De-Oiled Soya). J. Hazard. Mater. 2008, 151, 821–832. [Google Scholar] [CrossRef]
- Attia, A.A.; Rashwan, W.E.; Khedr, S.A. Capacity of activated carbon in the removal of acid dyes subsequent to its thermal treatment. Dye. Pigment. 2006, 69, 128–136. [Google Scholar] [CrossRef]
- Chen, X.; Lim, J.F.; Xu, Y.; Hong, L. Operating conditions and feed composition on filtering emulsified oil using ceramic-hybrid membrane. Ceram. Int. 2016, 42, 17101–17109. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Ramadhani, P.; Chaidir, Z.; Zilfa, Z.; Tomi, Z.B.; Rahmiarti, D.; Zein, R. Shrimp shell (Metapenaeus monoceros) waste as a low-cost adsorbent for metanil yellow dye removal in aqueous solution. Desalination Water Treat. 2020, 197, 413–423. [Google Scholar] [CrossRef]
- Khoshhesab, Z.M.; Souhani, S. Adsorptive removal of reactive dyes from aqueous solutions using zinc oxide nanoparticles. J. Chin. Chem. Soc. 2018, 65, 1482–1490. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Mallick, T.K. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Khitab, F. Sonophotocatalytic degradation of textile dyes over Cu impregnated ZnO catalyst in aqueous solution. Process Saf. Environ. Prot. 2018, 116, 149–158. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Jeon, Y.; Park, S.S.; Ha, C.-S. Hexadecyltrimethylammonium Bromide Surfactant-Supported Silica Material for the Effective Adsorption of Metanil Yellow Dye. ACS Omega 2019, 4, 8548–8558. [Google Scholar] [CrossRef]
- Slimani, R.; El Ouahabi, I.; Abidi, F.; El Haddad, M.; Regti, A.; Laamari, M.R.; El Antri, S.; Lazar, S. Calcined eggshells as a new biosorbent to remove basic dye from aqueous solutions: Thermodynamics, kinetics, isotherms and error analysis. J. Taiwan Inst. Chem. Eng. 2014, 45, 1578–1587. [Google Scholar] [CrossRef]
- Gong, R.; Sun, Y.; Chen, J.; Liu, H.; Yang, C. Effect of chemical modification on dye adsorption capacity of peanut hull. Dye. Pigment. 2005, 67, 175–181. [Google Scholar] [CrossRef]
- Akar, T.; Tosun, I.; Kaynak, Z.; Ozkara, E.; Yeni, O.; Sahin, E.N.; Akar, S.T. An attractive agro-industrial by-product in environmental cleanup: Dye biosorption potential of untreated olive pomace. J. Hazard. Mater. 2009, 166, 1217–1225. [Google Scholar] [CrossRef]
- Hameed, B. Spent tea leaves: A new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. J. Hazard. Mater. 2009, 161, 753–759. [Google Scholar] [CrossRef]
- Srivastava, R.; Rupainwar, D. A comparative evaluation for adsorption of dye on Neem bark and mango bark powder. Indian J. Chem. Technol. 2011, 18, 67–75. [Google Scholar]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.S.; Franca, A.S.; Alves, T.M.; Rocha, S.D. Evaluation of untreated coffee husks as potential biosorbents for treatment of dye contaminated waters. J. Hazard. Mater. 2008, 155, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Sadaf, S.; Bhatti, H.N. Batch and fixed bed column studies for the removal of Indosol Yellow BG dye by peanut husk. J. Taiwan Inst. Chem. Eng. 2014, 45, 541–553. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Agricultural peels for dye adsorption: A review of recent literature. J. Mol. Liq. 2014, 200, 381–389. [Google Scholar] [CrossRef]
- Wang, P.; Ma, Q.; Hu, D.; Wang, L. Adsorption of methylene blue by a low-cost biosorbent: Citric acid modified peanut shell. Desalin. Water Treat. 2016, 57, 10261–10269. [Google Scholar] [CrossRef]
- Wong, S.; Tumari, H.H.; Ngadi, N.; Mohamed, N.B.; Hassan, O.; Mat, R.; Amin, N.A.S. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J. Clean. Prod. 2018, 206, 394–406. [Google Scholar] [CrossRef]
- Okeowo, I.O.; Balogun, E.O.; Ademola, A.J.; Alade, A.O.; Afolabi, T.J.; Dada, E.O.; Farombi, A.G. Adsorption of Phenol from Wastewater Using Microwave-Assisted Ag–Au Nanoparticle-Modified Mango Seed Shell-Activated Carbon. Int. J. Environ. Res. 2020, 14, 215–233. [Google Scholar] [CrossRef]
- Luyen, N.T.; Linh, H.X.; Huy, T.Q. Preparation of Rice Husk Biochar-Based Magnetic Nanocomposite for Effective Removal of Crystal Violet. J. Electron. Mater. 2019, 49, 1142–1149. [Google Scholar] [CrossRef]
- Khan, M.A.; Al Othman, Z.A.; Kumar, M.; Ola, M.S.; Siddique, M.R. Biosorption potential assessment of modified pistachio shell waste for methylene blue: Thermodynamics and kinetics study. Desalination Water Treat. 2014, 56, 146–160. [Google Scholar] [CrossRef]
- Rafiee, A.; Nasab, S.G.; Teimouri, A. Synthesis and characterization of pistachio shell/nanodiopside nanocomposite and its application for removal of Crystal Violet dye from aqueous solutions using central composite design. Int. J. Environ. Anal. Chem. 2019, 100, 1624–1649. [Google Scholar] [CrossRef]
- Meng, F.; Wang, L.; Pei, M.; Guo, W.; Liu, G. Adsorption of metanil yellow from aqueous solution using polyaniline-bentonite composite. Colloid Polym. Sci. 2017, 295, 1165–1175. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Tural, S.; Tarhan, T.; Tural, B. Removal of hazardous azo dye Metanil Yellow from aqueous solution by cross-linked magnetic biosorbent; equilibrium and kinetic studies. Desalination Water Treat. 2015, 57, 13347–13356. [Google Scholar] [CrossRef]
- Khan, M.; Naseer, S.; Khan, M.; Nazir, R.; Badshah, A.; Adnan Shujah, S.; Parveen, A. Magnetic solid-phase extraction of Cd (II) from water samples using magnetic nanoparticles impregnated walnut shells powder (MNPS-WSP). Desalination Water Treat. 2021, 228, 286–296. [Google Scholar] [CrossRef]
- Shokoohi, R.; Torkshavand, Z.; Mahmoudi, M.M.; Behgoo, A.M.; Ghaedrahmati, E.; Hosseini, F.M. Effective Removal of Azo Dye Reactive Blue 222 from Aqueous Solutions Using Modified Magnetic Nanoparticles with Sodium Alginate/Hydrogen Peroxide. Environ. Prog. Sustain. Energy 2018, 38, S205–S213. [Google Scholar] [CrossRef]
- Nordin, A.H.; Wong, S.; Ngadi, N.; Zainol, M.M.; Latif, N.A.F.A.; Nabgan, W. Surface functionalization of cellulose with polyethyleneimine and magnetic nanoparticles for efficient removal of anionic dye in wastewater. J. Environ. Chem. Eng. 2020, 9, 104639. [Google Scholar] [CrossRef]
- Regti, A.; Laamari, M.R.; Stiriba, S.-E.; El Haddad, M. Removal of Basic Blue 41 dyes using Persea americana-activated carbon prepared by phosphoric acid action. Int. J. Ind. Chem. 2016, 8, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Salari, N.M.A.; Tehrani, R.; Motamedi, M. Zeolite modification with cellulose nanofiber/magnetic nanoparticles for the elimination of reactive red 198. Int. J. Biol. Macromol. 2021, 176, 342–351. [Google Scholar] [CrossRef]
- Aydın, H.; Baysal, G. Adsorption of acid dyes in aqueous solutions by shells of bittim (Pistacia khinjuk Stocks). Desalination 2006, 196, 248–259. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Evrendilek, G.A.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Nejadshafiee, V.; Islami, M.R. Adsorption capacity of heavy metal ions using sultone-modified magnetic activated carbon as a bio-adsorbent. Mater. Sci. Eng. C 2019, 101, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Majumder, C.B.; Kumar, S.; Sarkar, B. Removal of Direct Blue-86 dye from aqueous solution using alginate encapsulated activated carbon (PnsAC-alginate) prepared from waste peanut shell. J. Environ. Chem. Eng. 2019, 7, 103365. [Google Scholar] [CrossRef]
- Sivashankar, R.; Sathya, A.; Vasantharaj, K.; Sivasubramanian, V. Magnetic composite an environmental super adsorbent for dye sequestration—A review. Environ. Nanotechnol. Monit. Manag. 2014, 1–2, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Shan, R.R.; Yan, L.G.; Yang, K.; Yu, S.J.; Hao, Y.F.; Yu, H.Q.; Du, B. Magnetic Fe3O4/MgAl-LDH composite for effective removal of three red dyes from aqueous solution. Chem. Eng. J. 2014, 252, 38–46. [Google Scholar] [CrossRef]
- Nejati, K.; Rezvani, Z.; Mansurfar, M.; Mirzaee, A.; Mahkam, M. Adsorption of Metanil Yellow Azoic Dye from Aqueous Solution onto Mg-Fe-NO3 Layered Double Hydroxide. Z. Für Anorg. Allg. Chem. 2011, 637, 1573–1579. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Pawar, R.R.; Senthilkumar, S.; Somani, R.S.; Cho, M.H.; Bajaj, H.C. Pilot-scale produced super activated carbon with a nanoporous texture as an excellent adsorbent for the efficient removal of metanil yellow. Powder Technol. 2018, 333, 243–251. [Google Scholar] [CrossRef]
- Zein, R.; Tomi, Z.B.; Fauzia, S.; Zilfa, Z. Modification of rice husk silica with bovine serum albumin (BSA) for improvement in adsorption of metanil yellow dye. J. Iran. Chem. Soc. 2020, 17, 2599–2612. [Google Scholar] [CrossRef]






| Elements | PS | CPS | CPSMNPs |
|---|---|---|---|
| Oxygen (O) | 9.40 | 9.83 | 15.94 |
| Chlorine (Cl) | 39.56 | 1.52 | 0.00 |
| Iron (Fe) | 1.99 | 2.39 | 54.98 |
| Carbon (C) | 49.05 | 86.26 | 29.08 |
| Adsorbent | BET Surface Area (m2/g) |
|---|---|
| PS | 26.18 |
| CPS | 32.49 |
| CPSMNPs | 112.58 |
| Adsorbent | %Removal of MY | SD |
|---|---|---|
| PS | 63.32 | 6.36 |
| CPS | 94.78 | 7.32 |
| CPSMNPs | 94.83 | 1.21 |
| Isotherm | Parameters | Values |
|---|---|---|
| Freundlich isotherm | KF (mg g−1) | 28.24 |
| N | 0.946 | |
| 1/n | 0.5847 | |
| R2 | 0.983 | |
| Langmuir isotherm | aL (L mg−1) | 0.56 |
| KL (L g−1) | 54.6 | |
| Qo (mg g−1) | 97 | |
| R2 | 0.9947 |
| Kinetic Model | Parameters | Values |
|---|---|---|
| qe (exp) | 3433.96 | |
| Pseudo first order | K1 (min−1) | 0.2208 |
| qe calc (mg g−1) | 1.1365 | |
| R2 | 0.8297 | |
| C | 0.0556 | |
| Pseudo second order | K2 (min−1) | 0.0003 |
| qe calc (mg g−1) | 1428.57 | |
| qe2 calc (mg g−1) | 2,040,816.3 | |
| R2 | 0.9803 | |
| C | 0.0014 |
| Temperature (K) | −ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ K−1) |
|---|---|---|---|
| 313 | 30.430 | −23.670 | 0.022 |
| 323 | 31.091 | - | - |
| 333 | 31.831 | - | - |
| 343 | 31.490 | - | - |
| 353 | 31.880 | - | - |
| 363 | 31.570 | - | - |
| Adsorbents | Adsorption Capacity (mg.g−1) | Time (min) | pH | Reference |
|---|---|---|---|---|
| Leaves Prosopis juliflora | 26.00 | 150 | 2 | [11] |
| Mg-Fe-NO3 Layered double hydroxide | 42.72 | 120 | 6 | [56] |
| Pilot-scale produced super-activated carbon | 937.00 | 60 | 3 | [57] |
| Peanut shell-based activated carbon | 52.00 | 150 | 2 | [3] |
| Shrimp shell | 69.31 | 75 | 5 | [20] |
| Bovine serum albumin-modified rice husk silica | 80.00 | 120 | 5 | [58] |
| Saw dust carbon | 183.00 | 400 | 3 | [12] |
| Cross linked magnetic biosorbent | 385.00 | 240 | 6 | [44] |
| Polyaniline-bentonite composite (Bent) | 444.44 | 240 | 7 | [42] |
| CPSMNPs | 1428.00 | 40 | 2 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan; Omer, M.; Khan, B.; Khan, I.; Alamzeb, M.; Zada, F.M.; Ullah, I.; Shah, R.; Alqarni, M.; Simal-Gandara, J. Equilibrium, Kinetic and Thermodynamic Studies for the Adsorption of Metanil Yellow Using Carbonized Pistachio Shell-Magnetic Nanoparticles. Water 2022, 14, 4139. https://doi.org/10.3390/w14244139
Adnan, Omer M, Khan B, Khan I, Alamzeb M, Zada FM, Ullah I, Shah R, Alqarni M, Simal-Gandara J. Equilibrium, Kinetic and Thermodynamic Studies for the Adsorption of Metanil Yellow Using Carbonized Pistachio Shell-Magnetic Nanoparticles. Water. 2022; 14(24):4139. https://doi.org/10.3390/w14244139
Chicago/Turabian StyleAdnan, Muhammad Omer, Behramand Khan, Inkisar Khan, Muhammad Alamzeb, Farah Muhammad Zada, Ihsan Ullah, Rahim Shah, Mohammed Alqarni, and Jesus Simal-Gandara. 2022. "Equilibrium, Kinetic and Thermodynamic Studies for the Adsorption of Metanil Yellow Using Carbonized Pistachio Shell-Magnetic Nanoparticles" Water 14, no. 24: 4139. https://doi.org/10.3390/w14244139
APA StyleAdnan, Omer, M., Khan, B., Khan, I., Alamzeb, M., Zada, F. M., Ullah, I., Shah, R., Alqarni, M., & Simal-Gandara, J. (2022). Equilibrium, Kinetic and Thermodynamic Studies for the Adsorption of Metanil Yellow Using Carbonized Pistachio Shell-Magnetic Nanoparticles. Water, 14(24), 4139. https://doi.org/10.3390/w14244139