Oxidation of Aqueous Dexamethasone Solution by Gas-Phase Pulsed Corona Discharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Equipment and Procedure
2.3. Methods of Analysis
2.4. Identification of DXM Transformation Products
2.5. Acute Toxicity Test
3. Results and Discussion
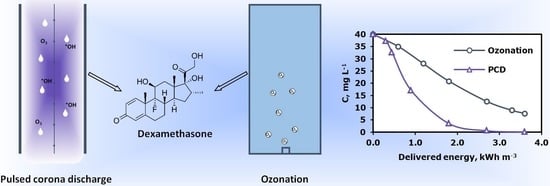
3.1. Oxidation Efficiency
3.2. Effect of Initial Concentration
3.3. Effect of pH
3.4. Effect of Radical-Scavenging Additives
3.5. Identification of Oxidation End-Products
3.6. Identification of Oxidation By-Products
3.7. Acute Toxicity of By-Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2011, 277, 111485. [Google Scholar] [CrossRef]
- Kostich, M.S.; Lazorchak, J.M. Risks to aquatic organisms posed by human pharmaceutical use. Sci. Total Environ. 2008, 389, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group, Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [CrossRef] [PubMed]
- Salas-Leiton, E.; Coste, O.; Arsensio, E.; Infante, C.; Canavate, J.P.; Manchado, M. Dexamethasone modulates expression of genes involved in the innate immune system, growth and stress and increases susceptibility to bacterial disease in Senegalese sole (Solea senegalensis Kaup, 1858). Fish Shellfish. Immunol. 2021, 32, 769–778. [Google Scholar] [CrossRef]
- Ribas, J.L.C.; Zampronio, A.R.; de Assis, H.C.S. Effects of trophic exposure to diclofenac and dexamethasone on hematological parameters and immune response in freshwater fish. Environ. Toxicol. Chem. 2016, 35, 975–982. [Google Scholar] [CrossRef]
- Chang, H.; Hu, J.; Shao, B. Occurrence of Natural and Synthetic Glucocorticoids in Sewage Treatment Plants and Receiving River Waters. Environ. Sci. Technol. 2007, 41, 3462–3468. [Google Scholar] [CrossRef]
- Macikova, P.; Groh, K.J.; Ammann, A.A.; Schirmer, K.; Suter, M.J.-F. Endocrine Disrupting Compounds Affecting Corticosteroid Signaling Pathways in Czech and Swiss Waters: Potential Impact on Fish. Environ. Sci. Technol. 2014, 48, 12902–12911. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Lin, C.; Xiong, X.; Chen, D.; Chen, Y.; Zhou, Y.; Wu, C.; Du, Y. Occurrence, distribution, and potential risks of environmental corticosteroids in surface waters from the Pearl River Delta, South China. Environ. Pollut. 2019, 251, 102–109. [Google Scholar] [CrossRef]
- Jia, A.; Wu, S.; Daniels, K.D.; Snyder, S.A. Balancing the Budget: Accounting for Glucocorticoid Bioactivity and Fate during Water Treatment. Environ. Sci. Technol. 2016, 50, 2870–2880. [Google Scholar] [CrossRef]
- Nakayama, K.; Sato, K.; Shibano, T.; Isobe, T.; Suzuki, G.; Kitamura, S.-I. Occurrence of glucocorticoids discharged from a sewage treatment plant in Japan and the effects of clobetasol propionate exposure on the immune responses of common carp (Cyprinus carpio) to bacterial infection. Environ. Toxicol. Chem. 2015, 35, 946–952. [Google Scholar] [CrossRef]
- Klauson, D.; Romero Sarcos, N.; Krichevskaya, M.; Kattel, E.; Dulova, N.; Dedova, T.; Trapido, M. Advanced oxidation processes for sulfonamide antibiotic sulfamethizole degradation: Process applicability study at ppm level and scale-down to ppb level. J. Environ. Chem. Eng. 2019, 7, 103287. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S. Photo-Fenton applied to the removal of pharmaceutical and other pollutants of emerging concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100458. [Google Scholar] [CrossRef]
- Olak-Kucharczyk, M.; Foszpańczyk, M.; Żyłła, R.; Ledakowicz, S. Photodegradation and ozonation of ibuprofen derivatives in the water environment: Kinetics approach and assessment of mineralization and biodegradability. Chemosphere 2022, 291, 132742. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Majumder, S.K.; Ghosh, P. Ozonation of diclofenac in a laboratory scale bubble column: Intermediates, mechanism, and mass transfer study. J. Water Process. Eng. 2021, 44, 102325. [Google Scholar] [CrossRef]
- Sun, S.; Ren, J.; Liu, J.; Rong, L.; Wang, H.; Xiao, Y.; Sun, F.; Mei, R.; Chen, C.; Su, X. Pyrite-activated persulfate oxidation and biological denitrification for effluent of biological landfill leachate treatment system. J. Environ. Manage 2022, 304, 114290. [Google Scholar] [CrossRef]
- Malik, M.A.; Ghaffar, A.; Malik, S.A. Water purification by electrical discharges. Plasma Sources Sci. Technol. 2001, 10, 82–91. [Google Scholar] [CrossRef]
- Bogaerts, A.; Neyts, E.; Gijbels, R.; van der Mullen, J. Gas discharge plasmas and their applications. Spectrochim. Acta Part B 2002, 57, 609–658. [Google Scholar] [CrossRef]
- Magureanu, M.; Bradu, C.; Parvulescu, V.I. Plasma processes for the treatment of water contaminated with harmful organic compounds. J. Phys. D Appl. Phys. 2018, 51, 313003. [Google Scholar] [CrossRef]
- Panorel, I.; Preis, S.; Kornev, I.; Hatakka, H.; Louhi-Kultanen, M. Oxidation of aqueous pharmaceuticals by pulsed corona discharge. Environ. Technol. 2013, 34, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Onga, L.; Boroznjak, R.; Kornev, I.; Preis, S. Oxidation of aqueous organic molecules in gas-phase pulsed corona discharge affected by sodium dodecyl sulphate: Explanation of variability. J. Electrostat. 2021, 111, 103581. [Google Scholar] [CrossRef]
- Derevshchikov, V.; Dulova, N.; Preis, S. Oxidation of ubiquitous aqueous pharmaceuticals with pulsed corona discharge. J. Electrost. 2021, 110, 103567. [Google Scholar] [CrossRef]
- Ono, R.; Oda, T. Dynamics of ozone and OH radicals generated by pulsed corona discharge in humid-air flow reactor measured by laser spectroscopy. J. Appl. Phys. 2003, 93, 5876. [Google Scholar] [CrossRef]
- Markic, M.; Cvetnic, M.; Ukic, S.; Kusic, H.; Bolanca, T.; Bozic, A.L. Influence of process parameters on the effectiveness of photooxidative treatment of pharmaceuticals. J. Environ. Sci. Heal. A 2018, 53, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Rasolevandi, T.; Naseri, S.; Azarpira, H.; Mahvi, A.H. Photo-degradation of dexamethasone phosphate using UV/Iodide process: Kinetics, intermediates, and transformation pathways. J. Mol. Liq. 2019, 295, 111703. [Google Scholar] [CrossRef]
- Calza, P.; Pelizzetti, E.; Brussino, M.; Baiocchi, C. Ion Trap Tandem Mass Spectrometry Study of Dexamethasone Transformation Products on Light Activated TiO2 Surface. J. Am. Soc. Mass Spectrom. 2001, 12, 1286–1295. [Google Scholar] [CrossRef] [Green Version]
- Ghenaatgar, A.; Tehrani, R.M.A.; Khadir, A. Photocatalytic degradation and mineralization of dexamethasone using WO3 and ZrO2 nanoparticles: Optimization of operational parameters and kinetic studies. J. Water Process. Eng. 2019, 32, 100969. [Google Scholar] [CrossRef]
- Rahmani, H.; Rahmani, K.; Rahmani, A.; Zare, M.-R. Removal of dexamethasone from aqueous solutions using sono-nanocatalysis process. Res. J. Environ. Sci. 2015, 9, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Guo, A.; Guo, Q.; Rui, M.; Zhao, Y.; Zhang, H.; Zhu, S. Decomposition of dexamethasone by gamma irradiation: Kinetics, degradation mechanisms and impact on algae growth. Chem. Eng. J. 2017, 307, 722–728. [Google Scholar] [CrossRef]
- Arsand, D.R.; Kümmerer, K.; Martins, A.F. Removal of dexamethasone from aqueous solution and hospital wastewater by electrocoagulation. Sci. Total Environ. 2013, 443, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Asgari, G.; Salari, M.; Faraji, H. Performance of heterogeneous catalytic ozonation process using Al2O3 nanoparticles in dexamethasone removal from aqueous solutions. Desalin. Water Treat. 2020, 189, 296–304. [Google Scholar] [CrossRef]
- Quaresma, A.V.; Rubio, K.T.S.; Taylor, J.G.; Sousa, B.A.; Silva, S.Q.; Werle, A.A.; Alfonso, R.J.C.F. Removal of dexamethasone by oxidative processes: Structural characterization of degradation products and estimation of the toxicity. J. Environ. Chem. Eng. 2021, 9, 106884. [Google Scholar] [CrossRef]
- Kahru, A.; Kurvet, M.; Külm, I. Toxicity of phenolic wastewater to luminescent bacteria photobacterium phosphoreum and activated sludges. Water Sci. Technol. 1996, 33, 139–146. [Google Scholar] [CrossRef]
- Preis, S.; Panorel, I.C.; Kornev, I.; Hatakka, H.; Kallas, J. Pulsed corona discharge: The role of ozone and hydroxyl radical in aqueous pollutants oxidation. Wat. Sci. Technol. 2013, 68, 1536–1542. [Google Scholar] [CrossRef]
- Wardman, P. Reduction potentials of one electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]
- Tikker, P.; Kornev, I.; Preis, S. Oxidation energy efficiency in water treatment with gas-phase pulsed corona discharge as a function of spray density. J. Electrost. 2020, 106, 103466. [Google Scholar] [CrossRef]
- Preis, S.; Panorel, I.C.; Llauger Coll, S.; Kornev, I. Formation of nitrates in aqueous solutions treated with pulsed corona discharge: The impact of organic pollutants. Ozone Sci. Eng. 2014, 36, 94–99. [Google Scholar] [CrossRef]
- Kask, M.; Krichevskaya, M.; Preis, S.; Bolobajev, J. Oxidation of aqueous N-nitrosodiethylamine: Experimental comparison of pulsed corona discharge with H2O2-assisted ozonation. J. Environ. Chem. Eng. 2021, 9, 105102. [Google Scholar] [CrossRef]
- Tikker, P.; Dulova, N.; Kornev, I.; Preis, S. Effects of persulfate and hydrogen peroxide on oxidation of oxalate by pulsed corona discharge. Chem. Eng. J. 2021, 411, 128586. [Google Scholar] [CrossRef]
- He, X.; Huang, H.; Tang, Y.; Guo, L. Kinetics and mechanistic study on degradation of prednisone acetate by ozone. J. Environ. Sci. Health A 2019, 55, 292–304. [Google Scholar] [CrossRef]
- Zhong, L.; Liang, Y.-Q.; Lu, M.; Pan, C.-G.; Dong, Z.; Zhao, H.; Li, C.; Lin, Z.; Yao, L. Effects of dexamethasone on the morphology, gene expression and hepatic histology in adult female mosquitofish (Gambusia affinis). Chemosphere 2021, 274, 129797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, K.; Hu, W.; Lu, X.; Li, L.; Zhang, Q.; Huang, H.; Wang, H. Prenatal dexamethasone exposure caused fetal rats liver dysplasia by inhibiting autophagy-mediated cell proliferation. Toxicology 2021, 449, 152664. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Fiorentino, A.; Isidori, M.; Lavorgna, M.; Previtera, L.; Rubino, M.; Temussi, F. Toxicity of prednisolone, dexamethasone and their photochemical derivatives on aquatic organisms. Chemosphere 2004, 54, 629–637. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onga, L.; Kattel-Salusoo, E.; Trapido, M.; Preis, S. Oxidation of Aqueous Dexamethasone Solution by Gas-Phase Pulsed Corona Discharge. Water 2022, 14, 467. https://doi.org/10.3390/w14030467
Onga L, Kattel-Salusoo E, Trapido M, Preis S. Oxidation of Aqueous Dexamethasone Solution by Gas-Phase Pulsed Corona Discharge. Water. 2022; 14(3):467. https://doi.org/10.3390/w14030467
Chicago/Turabian StyleOnga, Liina, Eneliis Kattel-Salusoo, Marina Trapido, and Sergei Preis. 2022. "Oxidation of Aqueous Dexamethasone Solution by Gas-Phase Pulsed Corona Discharge" Water 14, no. 3: 467. https://doi.org/10.3390/w14030467
APA StyleOnga, L., Kattel-Salusoo, E., Trapido, M., & Preis, S. (2022). Oxidation of Aqueous Dexamethasone Solution by Gas-Phase Pulsed Corona Discharge. Water, 14(3), 467. https://doi.org/10.3390/w14030467