Response Surface Modelling of Methylene Blue Adsorption onto Seaweed, Coconut Shell and Oak Wood Hydrochars
Abstract
:1. Introduction
2. Materials and Methods
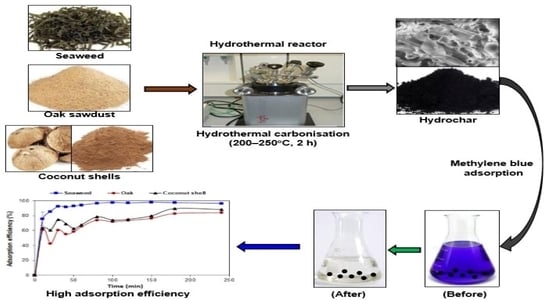
2.1. Raw Materials
2.2. Production of Hydrochars
2.3. Batch Adsorption Experiments
2.4. Response Surface Modelling and Optimisation
2.5. Calculations
2.5.1. Adsorption Kinetics
2.5.2. Adsorption Isotherms
3. Results and Discussion
3.1. Effect of Initial Methylene Blue Concentration on Adsorption
3.1.1. Adsorption Capacity
3.1.2. Removal Efficiency
3.2. Effect of pH on Adsorption Efficiency
3.3. Effect of Contact Time on Adsorption Efficiency
3.4. Adsorption Kinetics
3.5. Adsorption Isotherms
3.6. Proposed MB Removal Mechanisms
3.7. ANOVA, Modelling and Optimisation
3.7.1. Model Fitting and ANOVA
3.7.2. Optimisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1081. [Google Scholar] [CrossRef]
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.-H.; Rai, B.N.; Singh, R.S. Recent advancements in bioremediation of dye: Current status and challenges. Bioresour. Technol. 2018, 253, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Bhavani, R.; Sivasamy, A. Sonocatalytic degradation of malachite green oxalate by a semiconductor metal oxide nanocatalyst. Ecotoxicol. Environ. Saf. 2016, 134, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.U.; Paul, D.; Dhotre, D.; Kodam, K.M. Effective biotransformation and detoxification of anthraquinone dye reactive blue 4 by using aerobic bacterial granules. Water Res. 2017, 122, 603–613. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 1676–4697. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Mallick, T.K. A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Sun, K.; Ro, K.; Guo, M.; Novak, J.; Mashayekhi, H.; Xing, B. Sorption of bisphenol A, 17α-ethinyl estradiol and phenanthrene on thermally and hydrothermally produced biochars. Bioresour. Technol. 2011, 102, 5757–5763. [Google Scholar] [CrossRef] [PubMed]
- Mui, E.L.K.; Cheung, W.H.; Valix, M.; McKay, G. Dye adsorption onto char from bamboo. J. Hazard. Mater. 2010, 177, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-K.; Xiao, S.-C.; Yuan, J.-H.; Zhao, A.-Z. Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresour. Technol. 2011, 102, 10293–10298. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, X.; Wei, B.; Zhao, Y.; Wang, J. Evaluation of adsorption potential of bamboo biochar for metal-complex dye: Equilibrium, kinetics and artificial neural network modeling. Int J. Environ. Sci. Technol. 2014, 11, 1093–1100. [Google Scholar] [CrossRef]
- Dzyazko, Y.S.; Palchik, O.V.; Ogenko, V.M.; Shtemberg, L.Y.; Bogomaz, V.I.; Protsenko, S.A.; Khomenko, V.G.; Makeeva, I.S.; Chernysh, O.V.; Dzyazko, O.G. Nanoporous biochar for removal of toxic organic compounds from water. In Nanophotonics, Nanooptics, Nanobiotechnology, and Their Applications; Fesenko, O., Yatsenko, L., Eds.; Springer Proceedings in Physics, NANO 2018; Springer: New York, NY, USA, 2019; Volume 222. [Google Scholar] [CrossRef]
- Ramavandi, B.; Farjadfard, S. Removal of chemical oxygen demand from textile wastewater using a natural coagulant. Korean J. Chem. Eng. 2014, 31, 81–87. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, hysico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Jürgen Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management: Science and Technology, 1st ed.; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 13–32. [Google Scholar]
- Martin, M.J.; Artola, A.; Dolors Balaguer, M.; Rigola, M. Activated carbons developed from surplus sewage sludge for the removal of dyes from dilute aqueous solutions. Chem. Eng. J. 2003, 94, 231–239. [Google Scholar] [CrossRef]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X. Production and optimization of bamboo hydrochars for adsorption of Congo red and 2-naphthol. Bioresour. Technol. 2016, 207, 379–386. [Google Scholar] [CrossRef]
- Qian, W.C.; Luo, X.P.; Wang, X.; Guo, M.; Li, B. Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef]
- Li, B.; Wang, Q.; Guo, J.-Z.; Huan, W.-W.; Liu, K. Sorption of methyl orange from aqueous solution by protonated amine modified hydrochar. Bioresour. Technol. 2018, 268, 454–459. [Google Scholar] [CrossRef]
- Wu, F.-C.; Liu, B.-L.; Wu, K.-T.; Tseng, R.-L. A new linear form analysis of Redlich-Peterson isotherm equation for the adsorption of dyes. Chem. Eng. J. 2010, 162, 21–27. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, A.H.; Phama, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption isotherms and kinetic modeling of methylene blue dye onto a carbonaceous hydrochar adsorbent derived from coffee husk waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ji, Y.; Liu, X. Insights into interfacial interaction mechanism of dyes sorption on a novel hydrochar: Experimental and DFT study. Chem. Eng. Sci. 2021, 233, 116432. [Google Scholar] [CrossRef]
- Li, H.-Z.; Zhang, Y.-N.; Guo, J.-Z.; Lv, J.-Q.; Huan, W.-W.; Li, B. Preparation of hydrochar with high adsorption performance for methylene blue by co-hydrothermal carbonization of polyvinyl chloride and bamboo. Bioresour. Technol. 2021, 337, 125442. [Google Scholar] [CrossRef]
- Chung, J.W.; Edewi, O.C.; Foppen, J.W.; Gerner, G.; Krebs, R.; Lens, O.N.L. Removal of Escherichia coli by intermittent operation of saturated sand columns supplemented with hydrochar derived from sewage sludge. Appl. Sci. 2017, 7, 839. [Google Scholar] [CrossRef] [Green Version]
- Danso-Boateng, E.; Nyktari, E.; Wheatley, A.D.; Holdich, R.G.; Mohammed, A.S. Removal of organic pollutants from effluent of anaerobic digester using hydrochars produced from faecal simulant and sewage sludge. Water Air Soil Pollut. 2020, 231, 192. [Google Scholar] [CrossRef]
- Eljamal, O.; Sasaki, K.; Hirajima, T. Numerical simulation for reactive solute transport of arsenic in permeable reactive barrier column including zero-valent iron. Appl. Math. Model. 2011, 35, 5198–5207. [Google Scholar] [CrossRef]
- Maamoun, I.; Falyouna, O.; Eljamal, R.; Bensaida, K.; Eljamal, O. Optimization modeling of nFe0/Cu-PRB Design for Cr(VI) Removal from Groundwater. Int. J. Environ. Sci. Dev. 2021, 12, 131–138. [Google Scholar] [CrossRef]
- Eljamal, O.; Maamoun, I.; Alkhudhayri, S.; Eljamal, R.; Falyouna, O.; Tanaka, K.; Kozai, N.; Sugihara, Y. Insights into boron removal from water using Mg-Al-LDH: Reaction parameters optimization & 3D-RSM modeling. J. Water Process Eng. 2022, 46, 102608. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Mohammed, A.S.; Sander, G.; Wheatley, A.D.; Nyktari, E.; Usen, I.C. Production and characterisation of adsorbents synthesized by hydrothermal carbonisation of biomass wastes. SN Appl. Sci. 2021, 3, 257. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Greenway, G.M. Characterization of energy-rich hydrochars from microwave-assisted hydrothermal carbonization of coconut shell. Waste Biomass Valoriz. 2019, 10, 1979–1987. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Ross, A.B.; Mariner, T.; Hammerton, J.; Fitzsimmons, M. Hydrochars produced by hydrothermal carbonisation of seaweed, coconut shell and oak: Effect of processing temperature on physicochemical adsorbent characteristics. SN Appl. Sci. 2022, 4, 203. [Google Scholar] [CrossRef]
- Saha, N.; Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A.; Reza, T.M. Cationic dye adsorption on hydrochars of winery and citrus juice industries residues: Performance, mechanism, and thermodynamics. Energies 2020, 13, 4686. [Google Scholar] [CrossRef]
- Magat, S.S. Achieving coconut supply reliability through research-based crop nutrition management of coconut farms in the Philippines. CORD 2003, 19, 34. [Google Scholar] [CrossRef]
- Shahbandeh, M. Global Coconut Production 2000–2022. Available online: https://www.statista.com/statistics/577497/world-coconut-production/ (accessed on 11 October 2022).
- Ahmady-Asbchin, S.; Andrès, Y.; Gérente, C.; Cloirec, P. Biosorption of Cu (II) from aqueous solution by Fucus serratus: Surface characterization and sorption mechanisms. Bioresour. Technol. 2008, 99, 6150–6155. [Google Scholar] [CrossRef] [PubMed]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates from seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–274. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical constituents and biological activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Messineo, A.; Mäkelä, M.; Barr, M.R.; Volpe, R.; Corrado, C.; Fiori, L. Reactivity of cellulose during hydrothermal carbonization of lignocellulosic biomass. Fuel Process Technol. 2020, 206, 106456. [Google Scholar] [CrossRef]
- Borrero-lópez, A.M.; Masson, E.; Celzard, A.; Fierro, V. Modelling the reactions of cellulose, hemicellulose and lignin submitted to hydrothermal treatment. Ind. Crops Prod. 2018, 124, 919–930. [Google Scholar] [CrossRef]
- Mäkelä, M.; Volpe, M.; Volpe, R.; Fiori, L.; Dahl, O. Spatially resolved spectral determination of polysaccharides in hydrothermally carbonized biomass. Green Chem. 2018, 20, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Jawad, A.H.; Rashid, R.A.; Ishak, M.A.M.; Wilson, L.D. Adsorption of methylene blue onto activated carbon developed from biomass waste by H2SO4 activation: Kinetic, equilibrium and thermodynamic studies. Desalin. Water Treat. 2016, 57, 25194–25206. [Google Scholar] [CrossRef]
- Falyouna, O.; Bensaida, K.; Maamoun, I.; Ashik, U.P.M.; Tahara, A.; Tanaka, K.; Aoyagi, N.; Sugihara, Y.; Eljamal, O. Synthesis of hybrid magnesium hydroxide/magnesium oxide nanorods [Mg(OH)2/MgO] for prompt and efficient adsorption of ciprofloxacin from aqueous solutions. J. Clean. Prod. 2022, 342, 130949. [Google Scholar] [CrossRef]
- Martínez-Mendoza, K.L.; Barraza-Burgos, J.M.; Marriaga-Cabrales, N.; Machuca-Martinez, F.; Barajas, M.; Romero, M. Production and characterization of activated carbon from coal for gold adsorption in cyanide solutions. Ing. Investig. 2020, 40, 34–44. [Google Scholar] [CrossRef]
- Faust, D.S.; Aly, O.M. Chemistry of Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Arumugam, N.; Chelliapan, S.; Kamyab, H.; Thirugnana, S.; Othman, N.; Nasri, N.S. Treatment of wastewater using seaweed: A review. Int. J. Environ. Res. Public Health 2018, 15, 2851. [Google Scholar] [CrossRef] [Green Version]
- Jóźwiak, T.; Filipkowska, U.; Bugajska, P.; Kalkowski, T. The use of coconut shells for the removal of dyes from aqueous solutions. J. Ecol. Eng. 2018, 19, 129–135. [Google Scholar] [CrossRef]
- Liu, J.-L.; Qian, W.-C.; Guo, J.-Z.; Shen, Y.; Li, B. Selective removal of anionic and cationic dyes by magnetic Fe3O4-loaded amine-modified hydrochar. Bioresour. Technol. 2021, 320, 124374. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Coconut husk derived activated carbon via microwave induced activation: Effects of activation agents, preparation parameters and adsorption performance. Chem. Eng. J. 2012, 184, 57–65. [Google Scholar] [CrossRef]
- Lv, B.-W.; Xu, H.; Guo, J.-Z.; Bai, L.-Q.; Li, B. Efficient adsorption of methylene blue on carboxylate-rich hydrochar prepared by one-step hydrothermal carbonization of bamboo and acrylic acid with ammonium persulphate. J. Hazard. Mater. 2022, 421, 126741. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, C.D.; Pieris, M. A physico-chemical properties of coconut shell powder. Procedia Chem. 2015, 16, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. J. Environ. Manag. 2017, 203, 237–244. [Google Scholar] [CrossRef]
- Sangian, H.F.; Widjaja, A. The effect of alkaline concentration on coconut husk crystallinity and the yield of sugars released. IOP Conf. Ser. Mater. Sci. Eng. 2018, 306, 012046. [Google Scholar] [CrossRef]
- Floch, A.L.; Jourdes, M.; Teissedre, P.-L. Polysaccharides and lignin from oak wood used in cooperage: Composition, interest, assays: A review. Carbohydr. Res. 2015, 417, 94–102. [Google Scholar] [CrossRef]
- Navarro, A.E.; Portales, R.F.; Sun-Kou, M.R.; Llanos, B.P. Effect of pH on phenol biosorption by marine seaweeds. J. Hazard. Mater. 2008, 156, 405–411. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, Z.Y.; Su, X.P.; Chen, X.; Jiang, L.; Yao, J.M. Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent. ACS Sustain. Chem. Eng. 2015, 3, 432–442. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, B.; Cheng, R.; Li, Y. Behaviors and mechanism of acid dyes sorption onto diethylenetriamine-modified native and enzymatic hydrolysis starch. J. Hazard. Mater. 2010, 183, 224–232. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Jiang, Q.; Wang, L.; Zhang, Y. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2021, 401, 123292. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wu, X.; Xu, G.; Sun, Z.; Zheng, X.; Liu, J.; Tang, K. Tannin-immobilized cellulose microspheres as effective adsorbents for removing cationic dye (Methylene Blue) from aqueous solution. J. Chem. Technol. Biotechnol. 2017, 92, 1276–1284. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Li, J.; Zhu, Y.; Yu, H.; Peng, Y. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar. Chemosphere 2019, 236, 124254. [Google Scholar] [CrossRef]
- Batzias, F.A.; Sidiras, D.K. Dye adsorption by calcium chloride treated in batch and fixed bed systems. J. Hazard. Mater. 2004, 114, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Hydrothermal conversion of urban food waste to chars for removal of textile dyes from contaminated waters. Bioresour. Technol. 2014, 161, 310–319. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024–1026. [Google Scholar] [CrossRef]
- Dávila-Jiménez, M.M.; Elizalde-González, M.P.; García-Díaz, E.; González-Perea, M.; Guevara-Villa, M.R.G. Using Akaike Information Criterion to select the optimal isotherm equation for adsorption from solution. Adsorpt. Sci. Technol. 2014, 32, 605–622. [Google Scholar] [CrossRef]
- Melo, B.C.; Francisco, A.A.; Paulino, A.A.; Cardoso, V.A.; Pereira, A.G.B.; Fajardo, A.R.; Rodrigues, F.H.A. Cellulose nanowhiskers improve the methylene blue adsorption capacity of chitosan-g-poly(acrylic acid) hydrogel. Carbohydr. Polym. 2018, 181, 358–367. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmed, A.L.; Hameed, B.H. Adsorption of reactive dye onto cross-linked chitosan/oil palm ash composite beads. Chem. Eng. J. 2008, 136, 164–172. [Google Scholar] [CrossRef]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. The effect of augmentation of biochar and hydrochar in anaerobic digestion of a model substrate. Bioresour. Technol. 2021, 321, 124494. [Google Scholar] [CrossRef]










| Hydrochars | Pseudo Second-Order | Intraparticle Diffusion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k2 | qe | h | R2 | ki1 | C1 | R2 | Ki2 | C2 | R2 | Ki3 | C3 | R2 | |
| FS-HC200 | 4.93 | 0.98 | 4.69 | 1.00 | 0.36 | 2.64 | 1.00 | 0.10 | 3.98 | 0.99 | 0.01 | 4.80 | 0.36 |
| CS-HC200 | 4.46 | 0.01 | 0.001 | 0.99 | 0.90 | −1.92 | 1.00 | 0.42 | 0.01 | 0.99 | 0.12 | 2.38 | 0.92 |
| Oak-HC200 | 4.55 | 0.02 | 0.0002 | 0.99 | 0.23 | 2.32 | 0.51 | 0.21 | 1.87 | 0.52 | 0.15 | 2.19 | 0.83 |
| FS-HC220 | 4.98 | 0.09 | 0.04 | 1.00 | 0.29 | 2.18 | 0.94 | 0.02 | 4.51 | 0.68 | – | – | – |
| CS-HC220 | 4.39 | 0.03 | 0.001 | 1.00 | 0.01 | 3.26 | 0.03 | 0.08 | 3.07 | 0.84 | – | – | – |
| Oak-HC220 | 3.41 | 0.01 | 0.0002 | 0.98 | 0.40 | 0.001 | 0.93 | 0.12 | 1.42 | 0.74 | – | – | – |
| FS-HC250 | 4.46 | 13.25 | 784.18 | 0.99 | 0.01 | 4.43 | 0.31 | 0.22 | 1.35 | 0.80 | – | – | – |
| CS-HC250 | 4.39 | 0.94 | 3.86 | 1.00 | 0.28 | 2.49 | 0.96 | 0.08 | 3.56 | 0.86 | 0.03 | 4.00 | 0.82 |
| Oak-HC250 | 3.48 | 0.07 | 0.02 | 0.96 | 0.21 | 1.47 | 0.70 | 0.01 | 3.30 | 1.00 | – | – | – |
| Isotherm | Hydrochar | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| FS- HC200 | CS- HC200 | Oak- HC200 | FS- HC220 | CS- HC220 | Oak- HC220 | FS- HC250 | CS- HC250 | Oak- HC250 | |
| Freundlich | |||||||||
| n | 3.22 | 16.95 | 3.31 | 1.87 | 2.96 | 3.37 | 3.12 | 3.56 | 4.70 |
| KF (mg/g) | 3.85 | 12.59 | 2.72 | 3.18 | 1.08 | 1.09 | 3.79 | 2.58 | 3.62 |
| R2 | 0.59 | 0.02 | 0.61 | 0.64 | 0.95 | 0.70 | 0.62 | 0.36 | 0.57 |
| AIC | 13.27 | 14.55 | 10.30 | 35.54 | −3.79 | 5.80 | 13.31 | 17.04 | 9.49 |
| Langmuir | |||||||||
| b (L/mg) | 0.06 | 0.05 | 0.08 | 0.01 | 0.56 | 0.01 | 0.07 | 0.19 | 0.06 |
| qm (mg/g) | 8.62 | 5.10 | 6.94 | 28.57 | 10.10 | 10.10 | 8.55 | 5.16 | 5.62 |
| R2 | 0.88 | 0.96 | 0.99 | 0.34 | 0.93 | 0.56 | 0.96 | 0.83 | 0.86 |
| RL | 0.12 | 0.14 | 0.09 | 0.50 | 0.01 | 0.36 | 0.10 | 0.04 | 0.12 |
| AIC | 13.43 | 17.71 | 7.92 | 28.85 | 28.00 | 12.42 | 12.48 | 16.64 | 10.97 |
| Redlich–Peterson | |||||||||
| q’m | 1.94 | 3.06 | 1.56 | 0.62 | 1.08 | 1.00 | 1.91 | 1.58 | 1.94 |
| bRP (m3/g) | 5.16 | 16.36 | 32.04 | 16.02 | 9.27 | 9.98 | 5.23 | 6.35 | 5.15 |
| α | 0.70 | 0.94 | 0.70 | 0.47 | 0.70 | 0.71 | 0.69 | 0.73 | 0.81 |
| R2 | 0.87 | 0.86 | 0.89 | 0.58 | 0.99 | 0.93 | 0.88 | 0.79 | 0.95 |
| AIC | 23.20 | 24.49 | 20.28 | 23.24 | 14.26 | 15.90 | 23.67 | 27.02 | 19.63 |
| Conditions | % Dye Removal | ||
|---|---|---|---|
| FS-HC | CS-HC | Oak-HC | |
| Concentration modelling | |||
| 50 mg/L, 250 °C | 86.48 | 82.60 | |
| 50 mg/L, 200 °C | 91.73 | ||
| pH modelling | |||
| pH 6, 200 °C | 99.40 | ||
| pH 12, 250 °C | 80.22 | 93.42 | |
| Contact time modelling | |||
| 84 min, 200 °C | 98.85 | ||
| 190 min, 200 °C | 90.18 | ||
| 150 min, 250 °C | 95.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danso-Boateng, E.; Fitzsimmons, M.; Ross, A.B.; Mariner, T. Response Surface Modelling of Methylene Blue Adsorption onto Seaweed, Coconut Shell and Oak Wood Hydrochars. Water 2023, 15, 977. https://doi.org/10.3390/w15050977
Danso-Boateng E, Fitzsimmons M, Ross AB, Mariner T. Response Surface Modelling of Methylene Blue Adsorption onto Seaweed, Coconut Shell and Oak Wood Hydrochars. Water. 2023; 15(5):977. https://doi.org/10.3390/w15050977
Chicago/Turabian StyleDanso-Boateng, Eric, Melissa Fitzsimmons, Andrew B. Ross, and Ted Mariner. 2023. "Response Surface Modelling of Methylene Blue Adsorption onto Seaweed, Coconut Shell and Oak Wood Hydrochars" Water 15, no. 5: 977. https://doi.org/10.3390/w15050977
APA StyleDanso-Boateng, E., Fitzsimmons, M., Ross, A. B., & Mariner, T. (2023). Response Surface Modelling of Methylene Blue Adsorption onto Seaweed, Coconut Shell and Oak Wood Hydrochars. Water, 15(5), 977. https://doi.org/10.3390/w15050977