Assessing the Role of Kettle Holes for Providing and Connecting Amphibian Habitats in Agricultural Landscapes
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
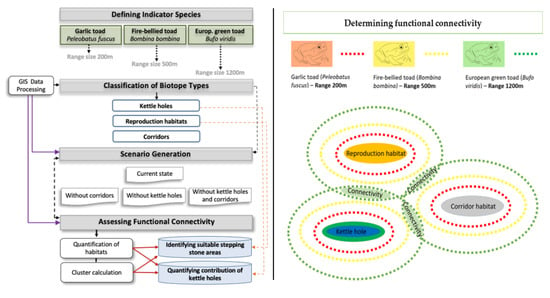
2.2. Indicator Species
2.3. Classification of Biotope Types
2.4. Assessing the Contribution of Kettle Holes to Amphibian Habitat Provision and Habitat Connectivity
2.4.1. Contribution of Kettle Holes to Habitat Provision
2.4.2. Calculating Clusters of Functionally Connected Habitats
2.4.3. Scenarios for Assessing the Contribution of Kettle Holes to Habitat Connectivity
2.5. Identification of Potential Areas for Artificial Stepping Stone Biotopes
3. Results
3.1. Importance of Kettle Holes for Amphibian Habitat Provision
3.2. Importance of Kettle Holes for Habitat Connectivity
3.3. Identifying Priority Areas for Improving Functional Connectivity
4. Discussion
4.1. Amphibians and Functional Connectivity
4.2. Methods for Measuring Functional Connectivity
4.3. Contribution of Kettle Holes to Habitat Provision and Habitat Connectivity
- Habitat Provision
- Habitat Connectivity
- Ecosystem Services
4.4. Climate Change in Relation to Habitat Provision and Connectivity by Kettle Holes
4.5. Decision Support for Landscape and Spatial Planning
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Reproduction Habitats
| BIOTYP Code | BIOTYP8 Code | BIOTYP8_T (Text_Description) |
| 02100 | 02100000 | Lakes |
| 02130 | 02130000 | Temporary small water bodies |
| 02140 | 02140000 | Water reservoirs |
| 02150 | 02150000 | Ponds |
| 02161 | 02161000 | Waters in peat ditches |
| 02200 | 02200000 | Floating leaf and submerged plant communities in standing waters. |
| 02210 | 02210000 | Reed communities at standing waters |
| 02211 | 02211000 | Tall reed fens at standing waters |
| 022111 | 02211100 | Tall reed fens on standing waters; reed canary grass |
| 022112 | 02211200 | Tall reed fens on standing waters; Cat-tail reed-beds. |
| 0513101 | 05131010 | Grassland fallows of moist sites; largely without spontaneous woody vegetation (<10% woody cover). |
Appendix A.2. Kettle Holes
| BIOTYP Code | BIOTYP8 Code | BIOTYP8_T (Text_Description) |
| 02120 | 02120000 | Perennial small water bodies (kettle holes potholes, mudholes, etc., <1 ha) |
Appendix A.3. Corridor Habitats
| BIOTYP Code | BIOTYP8 Code | BIOTYP8_T (Text_Description) |
| 05150 | 05150000 | Intensive grassland incl. intensive pastures |
| 051601 | 05160100 | Ornamental lawn/shear grass; largely without trees. |
| 051602 | 05160200 | Ornamental lawn/shear grass; with loosely standing trees. |
| 0510001 | 05100010 | Wet meadows and wet pastures; largely without spontaneous woody vegetation (<10% woody cover). |
| 0510101 | 05101010 | Tall sedge meadows (litter meadows); largely without spontaneous woody vegetation (<10% woody cover). |
| 0510301 | 05103010 | Wet meadows of nutrient-rich sites; largely without spontaneous woody vegetation (<10% woody cover). |
| 0510501 | 05105010 | Wet pastures; largely without spontaneous woody vegetation(<10% woody cover). |
| 0511001 | 05110010 | Mesotrophic grasslands; largely without spontaneous woody vegetation(<10% woody cover). |
| 0511002 | 05110020 | Mesotrophic grasslands with spontaneous woody vegetation(10–30% woody cover). |
| 0511101 | 05111010 | Mesotrophic pastures, fat pastures; largely without spontaneous woody vegetation (<10% woody cover). |
| 0511102 | 05111020 | Mesotrophic pastures, fat pastures; with spontaneous woody vegetation (10–30% woody cover). |
| 0511201 | 05112010 | Mesotrophic meadows; largely without spontaneous woody vegetation(<10% woody cover). |
| 0511202 | 05112020 | Mesotrophic meadows; with spontaneous woody vegetation(10–30% woody cover). |
| 0513001 | 05130010 | Grassland fallows; largely without spontaneous woody vegetation(<10% woody cover). |
| 0513002 | 05130020 | Grassland fallow; with spontaneous woody vegetation(10–30% woody cover). |
| 0513101 | 05131010 | Grassland fallows of moist sites; largely without spontaneous woody vegetation (<10% woody cover). |
| 0513102 | 05131020 | Grassland fallows of moist locations; with spontaneous woody vegetation(10–30% woody cover). |
| 0513111 | 05131110 | Grassland fallows of moist sites; dominated by reeds; largely without spontaneous woody vegetation (<10% woody cover). |
| 0513112 | 05131120 | Grassland fallows of wet sites; dominated by reeds; with spontaneous woody vegetation (10–30% woody cover). |
| 0513201 | 05132010 | Grassland fallows of mesotrophic sites; largely without spontaneous woody vegetation (<10% woody cover). |
| 0513202 | 05132020 | Grassland fallows of mesotrophic sites; with spontaneous woody vegetation (10–30% woody cover). |
| 0513301 | 05133010 | Grassland fallows of dry sites; largely without spontaneous woody vegetation (<10% woody cover). |
| 0513302 | 05133020 | Grassland fallows of dry locations; with spontaneous woody vegetation(10–30% woody cover). |
| 0514001 | 05140010 | Herbaceous meadows and shrublands; largely without spontaneous woody vegetation (<10% woody cover). |
| 0514101 | 05141010 | Tall forb communities of moist to wet sites; largely without spontaneous woody vegetation (<10% woody vegetation cover). |
| 0514102 | 05141020 | Tall forb communities of moist to wet sites; with spontaneous woody vegetation (10–30% woody vegetation cover) |
| 0514201 | 05142010 | Herbaceous vegetation (field margins) of fresh, nutrient-rich sites; largely without spontaneous woody vegetation (<10% woody vegetation cover). |
| 0514202 | 05142020 | Herbaceous meadows (field margins) of fresh, nutrient-rich sites; with spontaneous woody vegetation (10–30% woody vegetation cover) |
| 05120001 | 05120001 | Dry grasslands; largely without spontaneous woody vegetation(<10% woody cover). |
Appendix A.4. Dataset
Appendix A.5. Habitat Type Classification
Appendix B
Appendix B.1. Starting Point
- Kettle holes: containing polygon data and point data;
- Reproduction habitats: containing polygon data;
- Corridor habitats: containing polygon data.
Appendix B.2. Assigning Buffers
- 100 m for garlic toad;
- 250 m for fire-bellied toad;
- 600 m for European green toad.
Appendix B.3. Scenario Preparation
- Current state: all three layers were merged;
- Without kettle holes: the layers reproduction habitats and corridor habitats were merged;
- Without corridors: the layers reproduction habitats and kettle holes were merged;
- Without kettle holes and without corridor habitats: only the layer reproduction habitats was used, and no merge was necessary.
Appendix B.4. Calculating Clusters
Appendix B.4.1. Combining Polygons
Appendix B.4.2. Discarding Clusters without Reproduction Habitats
Appendix B.5. Identifying Areas Where the Creation of Stepping-Stone Habitats Could Improve Functional Connectivity
Appendix B.5.1. Re-Clustering
Appendix B.5.2. Assigning Buffers
Appendix B.5.3. Identifying Areas Where Buffers Overlap
References
- Pätzig, M.; Kalettka, T.; Glemnitz, M.; Berger, G. What governs macrophyte species richness in kettle hole types? A case study from Northeast Germany. Limnologica 2012, 42, 340–354. [Google Scholar] [CrossRef]
- Platen, R.; Kalettka, T.; Ulrichs, C. Kettle holes in the agrarian landscape: Isolated and ecological unique habitats for carabid beetles (col.: Carabidae) and spiders (arach.: Araneae). J. Landsc. Ecol. 2016, 9, 29–30. [Google Scholar] [CrossRef] [Green Version]
- Vasić, F.; Paul, C.; Strauss, V.; Helming, K. Ecosystem services of kettle holes in agricultural landscapes. Agronomy 2020, 10, 1326. [Google Scholar] [CrossRef]
- Lischeid, G.; Kalettka, T. Grasping the heterogeneity of kettle hole water quality in Northeast Germany. Hydrobiologia 2012, 689, 63–77. [Google Scholar] [CrossRef]
- Kalettka, T.; Berger, G.; Pfeffer, H.; Rudat, C. Integrated conservation and management of kettle holes in young moraine agricultural landscapes of northeast Germany. In Proceedings of the ICID 21st European Regional Conference, Frankfurt (Oder), Germany, Slubice, Poland, 15–19 May 2005; pp. 1–4. [Google Scholar]
- Arponen, A.; Heikkinen, R.K.; Paloniemi, R.; Pöyry, J.; Similä, J.; Kuussaari, M. Improving conservation planning for semi-natural grasslands: Integrating connectivity into agri-environment schemes. Biol. Conserv. 2013, 160, 234–241. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Heim, O.; Lorenz, L.; Kramer-Schadt, S.; Jung, K.; Voigt, C.C.; Eccard, J.A. Landscape and scale-dependent spatial niches of bats foraging above intensively used arable fields. Ecol. Process. 2017, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Baum, K.A.; Haynes, K.J.; Dillemuth, F.P.; Cronin, J.T. The matrix enhances the effectiveness of corridors and stepping stones. Ecology 2004, 85, 2671–2676. [Google Scholar] [CrossRef]
- Thiere, G.; Milenkovski, S.; Lindgren, P.E.; Sahlen, G.; Berglund, O.; Weisner, S.E.B. Wetland creation in agricultural landscapes: Biodiversity benefits on local and regional scales. Biol. Conserv. 2009, 142, 964–973. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, L.; Baccaro, F.B.; Ferreira, E.B.; Sampaio, M.F.D.O.; Santos, T.; Justino, R.C.; Angulo, A. The matrix effect: How agricultural matrices shape forest fragment structure and amphibian composition. J. Biogeogr. 2017, 44, 1911–1922. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Böhm, M.; Brooks, T.M.; Butchart, S.H.M.; Carpenter, K.E.; Chanson, J.; Collen, B.; Cox, N.A.; et al. The impact of conservation on the status of the world’s vertebrates. Science 2010, 330, 1503–1509. [Google Scholar] [CrossRef] [Green Version]
- Berger, G.; Pfeffer, H.; Kalettka, T. Amphibienschutz in Kleingewässerreichen Ackerbaugebieten; Nature + Text: Rangsdorf, Germany, 2011; p. 381. [Google Scholar]
- Hamm, M.; Drossel, B. Habitat heterogeneity hypothesis and edge effects in model metacommunities. J. Theor. Biol. 2017, 426, 40–48. [Google Scholar] [CrossRef]
- Meiklejohn, K.; Ament, R.; Tabor, G. Habitat Corridors & Landscape Connectivity: Clarifying the Terminology; Center for Large Landscape Conservation: Bozeman, MT, USA, 2010; Available online: https://tinyurl.com/28wcr3s (accessed on 16 February 2021).
- Herrera, L.P.; Sabatino, M.C.; Jaimes, F.R.; Saura, S. Landscape connectivity and the role of small habitat patches as stepping stones: An assessment of the grassland biome in South America. Biodivers. Conserv. 2017, 26, 3465–3479. [Google Scholar] [CrossRef]
- Taylor, P.D.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity is a vital element of landscape structure. Oikos 1993, 68, 571–573. [Google Scholar] [CrossRef] [Green Version]
- Bunn, A.G.; Urban, D.L.; Keitt, T.H. Landscape connectivity: A conservation application of graph theory. J. Environ. Manag. 2000, 59, 265–278. [Google Scholar] [CrossRef] [Green Version]
- McRae, B.H.; Beier, P. Circuit theory predicts gene flow in plant and animal populations. Proc. Natl. Acad. Sci. USA 2007, 104, 19885–19890. [Google Scholar] [CrossRef] [Green Version]
- Churko, G.; Kienast, F.; Bolliger, J. A multispecies assessment to identify the functional connectivity of amphibians in a human-dominated landscape. ISPRS Int. J. Geo-Inf. 2020, 9, 287. [Google Scholar] [CrossRef]
- Scholz, E. Die Naturräumliche Gliederung Brandenburgs; Pädagogisches Bezirkskabinett: Potsdam, Germany, 1962; p. 92. (In German) [Google Scholar]
- Köstler, H.; Grabowski, C.; Moeck, M.; Saure, C.; Kielhorn, K.-H. Beschreibung der Biotoptypen auf der Grundlage der Liste der Biotoptypen Brandenburgs (Stand 2004) und der Erläuterungstexte (Stand 1994) von Dr. Frank Zimmermann (Landesumweltamt Brandenburg); Arbeitsgemeinschaft Biotopkartierung: Berlin, Germany, 2005; Available online: https://tinyurl.com/4xbadf86 (accessed on 29 June 2021).
- Amler, K.; Bahl, A.; Henle, K.; Kaule, G.; Poschlod, P.; Settele, J. Populationsbiologie in der Naturschutzpraxis. Isolation, Flächenbedarf und Biotopansprüche von Pflanzen und Tieren; Ulmer: Stuttgart, Germany, 1999. [Google Scholar]
- Meinig, H.; Boye, P.; Dähne, M.; Hutterer, R.; Lang, J. Rote Liste und Gesamtartenliste der Säugetiere (Mammalia) Deutschlands. Band 170 (2): Säugetiere; Bundesamt für Naturschutz: Bonn, Germany, 2020; ISBN 978-3-7843-3772-2. [Google Scholar]
- MLUL (2014) Ministerium für Ländliche Entwicklung, Umwelt und Landwirtschaft des Landes Brandenburg (MLUL). Flächendeckende Biotop- und Landnutzungskartierung (BTLN) im Land Brandenburg-CIR-Biotoptypen 2009, dl-de/by-2-0. Potsdam. Available online: https://tinyurl.com/xbcm9rvt (accessed on 4 June 2021).
- LfU. Brandenburg State Office for the Environment (2013): Documentation of the Brandenburg Land Use and Biotope Mapping 2009. Available online: https://tinyurl.com/fby8jypk (accessed on 19 March 2021).
- Hoffmann, J.; Wittchen, U.; Wahrenberg, T. Hydrological situation of small water bodies and their avifauna in arable farming areas in eastern Brandenburg with reference to meteorological conditions and yield development. Nat. Landsch.Brandenbg. 2020, 29, 24–45. (In German) [Google Scholar]
- Ficetola, G.F.; De Bernardi, F. Amphibians in a human-dominated landscape: The community structure is related to habitat features and isolation. Biol. Conserv. 2004, 119, 219–230. [Google Scholar] [CrossRef]
- Cushman, S.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Abrahams, C.; Meilink, W.R.M.; Iosif, R.; Zuiderwijk, A. Amphibian decline, pond loss and reduced population connectivity under agricultural intensification over a 38 year period. Biodivers. Conserv. 2017, 26, 1411–1430. [Google Scholar] [CrossRef]
- Pittman, S.E.; Osbourn, M.S.; Semlitsch, R.D. Movement ecology of amphibians: A missing component for understanding population declines. Biol. Conserv. 2014, 169, 44–53. [Google Scholar] [CrossRef]
- Carroll, C.; Miquelle, D.G. Spatial viability analysis of Amur tiger Panthera tigris altaica in the Russian Far East: The role of protected areas and landscape matrix in population persistence. J. Appl. Ecol. 2006, 43, 1056–1068. [Google Scholar] [CrossRef]
- LaRue, M.A.; Nielsen, C.K. Modelling potential dispersal corridors for cougars in midwestern North America using least-cost path methods. Ecol. Model. 2008, 212, 372–381. [Google Scholar] [CrossRef]
- McRae, B. Isolation by resistance. Evol. Int. J. Org. Evol. 2006, 60, 1551–1561. [Google Scholar] [CrossRef]
- Minor, E.S.; Urban, D.L. A graph-theory frarmework for evaluating landscape connectivity and conservation planning. Conserv. Biol. 2008, 22, 297–307. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 2008, 89, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- Urban, D.; Keitt, T. Landscape connectivity: A graph-theoretic perspective. Ecology 2001, 82, 1205–1218. [Google Scholar] [CrossRef]
- Emel, S.L.; Wang, S.; Metz, R.P.; Spigler, R.B. Type and intensity of surrounding human land use, not local environment, shape genetic structure of a native grassland plant. Mol. Ecol. 2021, 30, 639–655. [Google Scholar] [CrossRef]
- Kalettka, T.; Rudat, C. Hydrogeomorphic types of glacially created kettle holes in North-East Germany. Limnologica 2006, 36, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Acreman, M.C.; Mccartney, M.P. Hydrological Impacts in and around wetlands. In The Wetlands Handbook; Maltby, E., Barker, T., Eds.; Wiley-Blackwell: Chichester, UK, 2009; pp. 643–666. [Google Scholar]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.; Greenwood, M.; Agnew, M. Pond Biodiversity and Habitat Loss in the UK. Area 2003, 35, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Milanovich, J.R.; Peterman, W.E.; Nibbelink, N.P.; Maerz, J.C. Projected loss of a salamander diversity hotspot as a consequence of projected global climate change. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zank, C.; Becker, F.G.; Abadie, M.; Baldo, D.; Maneyro, R.; Borges-Martins, M. Climate change and the distribution of neotropical red-bellied toads (Melanophryniscus, Anura, Amphibia): How to prioritize species and populations? PLoS ONE 2014, 9, e94625. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.; Thuiller, W.; Pearson, R. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- McKelvey, K.S.; Copeland, J.P.; Schwartz, M.K.; Littell, J.S.; Aubry, K.B.; Squires, J.R.; Parks, S.A.; Elsner, M.M.; Mauger, G.S. Climate change predicted to shift wolverine distributions, connectivity, and dispersal corridors. Ecol. Appl. 2011, 21, 2882–2897. [Google Scholar] [CrossRef] [Green Version]
- Kool, J.T.; Moilanen, A.; Treml, E.A. Population connectivity: Recent advances and new perspectives. Landsc. Ecol. 2013, 28, 165–185. [Google Scholar] [CrossRef]
- Hodgson, J.A.; Thomas, C.D.; Wintle, B.A.; Moilanen, A. Climate change, connectivity and conservation decision making: Back to basics. J. Appl. Ecol. 2009, 46, 964–969. [Google Scholar] [CrossRef]
- Heller, N.E.; Zavaleta, E.S. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol. Conserv. 2009, 142, 14–32. [Google Scholar] [CrossRef]
- Hannah, L.; Flint, L.; Syphard, A.D.; Moritz, M.A.; Buckley, L.B.; McCullough, I.M. Fine-grain modeling of species’ response to climate change: Holdouts, stepping-stones, and microrefugia. Trends Ecol. Evol. 2014, 29, 390–397. [Google Scholar] [CrossRef]
- Anderson, M.G.; Comer, P.J.; Beier, P.; Lawler, J.J.; Schloss, C.A.; Buttrick, S.; Albano, C.M.; Faith, D.P. Case studies of conservation plans that incorporate geodiversity. Conserv. Biol. 2015, 29, 680–691. [Google Scholar] [CrossRef]
- SWD. Commission Staff Working Document. EU Guidance on Integrating Ecosystems and Their Services into Decision-Making; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Ayram, C.A.C.; Mendoza, M.E.; Etter, A.; Salicrup, D.R.P. Habitat connectivity in biodiversity conservation: A review of recent studies and applications. Prog. Phys. Geogr. Earth Environ. 2016, 40, 7–37. [Google Scholar] [CrossRef]
- Grêt-Regamey, A.; Siren, E.; Brunner, S.H.; Weibel, B. Review of decision support tools to operationalize the ecosystem services concept. Ecosyst. Serv. 2017, 26, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Maes, J.; Egoh, B.; Willemen, L.; Liquete, C.; Vihervaara, P.; Schagner, J.P.; Grizzetti, B.; Drakou, E.G.; La Notte, A.; Zulian, G.; et al. Mapping ecosystem services for policy support and decision making in the European Union. Ecosyst. Serv. 2012, 1, 31–39. [Google Scholar] [CrossRef]






| Specifications | Garlic Toad Peleobatus fuscus | Fire-Bellied Toad Bombina bombina | European Green Toad Bufo viridis |
|---|---|---|---|
| Appearance 1,2,3 |  |  |  |
| Movement Range (m) | Short range 200-500 | Medium range 500-800 | Long range 1200-1500 |
| Movement Range used for this Research (m) | 200 | 500 | 1200 |
| Endangerment and Protection | Severely endangered/specially protected | Threatened with extinction/strictly protected | Threatened with extinction/strictly protected |
| Study Sites | Kettle Holes | Reproduction Habitats (Excluding Kettle Holes) | Corridor Habitats | |||
|---|---|---|---|---|---|---|
| Total Area (ha) | Count | Total Area (ha) | Count | Total Area (ha) | Count | |
| Uckermark | 56.4 | 490 | 17.8 | 60 | 1267.6 | 743 |
| Märkisch-Oderland | 29.2 | 254 | 38.3 | 69 | 1264.8 | 750 |
| Prignitz | 26.3 | 229 | 52.1 | 32 | 495.7 | 526 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savić, B.; Evgrafova, A.; Donmez, C.; Vasić, F.; Glemnitz, M.; Paul, C. Assessing the Role of Kettle Holes for Providing and Connecting Amphibian Habitats in Agricultural Landscapes. Land 2021, 10, 692. https://doi.org/10.3390/land10070692
Savić B, Evgrafova A, Donmez C, Vasić F, Glemnitz M, Paul C. Assessing the Role of Kettle Holes for Providing and Connecting Amphibian Habitats in Agricultural Landscapes. Land. 2021; 10(7):692. https://doi.org/10.3390/land10070692
Chicago/Turabian StyleSavić, Biljana, Alevtina Evgrafova, Cenk Donmez, Filip Vasić, Michael Glemnitz, and Carsten Paul. 2021. "Assessing the Role of Kettle Holes for Providing and Connecting Amphibian Habitats in Agricultural Landscapes" Land 10, no. 7: 692. https://doi.org/10.3390/land10070692
APA StyleSavić, B., Evgrafova, A., Donmez, C., Vasić, F., Glemnitz, M., & Paul, C. (2021). Assessing the Role of Kettle Holes for Providing and Connecting Amphibian Habitats in Agricultural Landscapes. Land, 10(7), 692. https://doi.org/10.3390/land10070692