Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes
Abstract
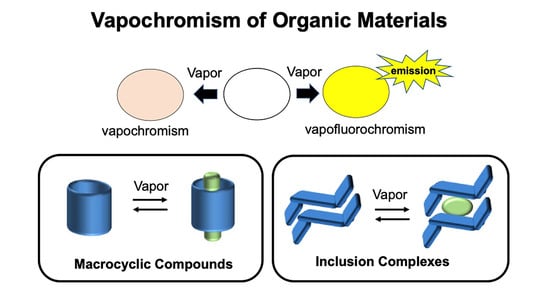
:1. Introduction
2. Vapochromic Materials Based on Macrocyclic Compounds
3. Vapochromic Materials Based on Inclusion Crystals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Chen, Z.-H.; Chen, Z.-N. Luminescence vapochromism in solid materials based on metal complexes for detection of volatile organic compounds (VOCs). J. Mater. Chem. 2012, 22, 11427–11441. [Google Scholar] [CrossRef]
- Wenger, O.S. Vapochromism in organometallic and coordination complexes: Chemical sensors for volatile organic compounds. Chem. Rev. 2013, 113, 3686–3733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent Progress on the Development of Chemosensors for Gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ito, H.; Hasegawa, M.; Ishii, K. Soft Crystals: Flexible Response Systems with High Structural Order. Chem. Eur. J. 2019, 25, 5105–5112. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Jie, K.; Liu, M.; Sheng, X.; Zhu, W.; Huang, F. Vapochromic crystals: Understanding vapochromism from the perspective of crystal engineering. Chem. Soc. Rev. 2020, 49, 1517–1544. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Ikeda, A.; Shinkai, S. Novel Cavity Design Using Calix[n]arene Skeletons: Toward Molecular Recognition and Metal Binding. Chem. Rev. 1997, 97, 1713–1734. [Google Scholar] [CrossRef]
- Morohashi, N.; Narumi, F.; Iki, N.; Hattori, T.; Miyano, S. Thiacalixarenes. Chem. Rev. 2006, 106, 5291–5316. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T.-A.; Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar [n] arenes: New key players for supramolecular chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Lu, F.; Wang, J.; Hu, W.; Cao, X.M.; Ma, X.; Tian, H. Amorphous Metal-Free Room-Temperature Phosphorescent Small Molecules with Multicolor Photoluminescence via a Host-Guest and Dual-Emission Strategy. J. Am. Chem. Soc. 2018, 140, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Pirondini, L.; Dalcanale, E. Molecular recognition at the gas–solid interface: A powerful tool for chemical sensing. Chem. Soc. Rev. 2007, 36, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-A.; Nakamoto, Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Ogoshi, T.; Tsuchida, H.; Kakuta, T.; Yamagishi, T.A.; Taema, A.; Ono, T.; Sugimoto, M.; Mizuno, M. Ultralong Room-Temperature Phosphorescence from Amorphous Polymer Poly(Styrene Sulfonic Acid) in Air in the Dry Solid State. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Jie, K.; Zhou, Y.; Li, E.; Huang, F. Nonporous Adaptive Crystals of Pillararenes. Acc. Chem. Res. 2018, 51, 2064–2072. [Google Scholar] [CrossRef]
- Jie, K.; Liu, M.; Zhou, Y.; Little, M.A.; Bonakala, S.; Chong, S.Y.; Stephenson, A.; Chen, L.; Huang, F.; Cooper, A.I. Styrene Purification by Guest-Induced Restructuring of Pillar[6]arene. J. Am. Chem. Soc. 2017, 139, 2908–2911. [Google Scholar] [CrossRef]
- Jie, K.; Liu, M.; Zhou, Y.; Little, M.A.; Pulido, A.; Chong, S.Y.; Stephenson, A.; Hughes, A.R.; Sakakibara, F.; Ogoshi, T.; et al. Near-Ideal Xylene Selectivity in Adaptive Molecular Pillar[n]arene Crystals. J. Am. Chem. Soc. 2018, 140, 6921–6930. [Google Scholar] [CrossRef]
- Kakuta, T.; Yamagishi, T.-A.; Ogoshi, T. Stimuli-Responsive Supramolecular Assemblies Constructed from Pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef]
- Ogoshi, T.; Shimada, Y.; Sakata, Y.; Akine, S.; Yamagishi, T.-A. Alkane-Shape-Selective Vapochromic Behavior Based on Crystal-State Host–Guest Complexation of Pillar[5]arene Containing One Benzoquinone Unit. J. Am. Chem. Soc. 2017, 139, 5664–5667. [Google Scholar] [CrossRef]
- Wada, K.; Kakuta, T.; Yamagishi, T.-A.; Ogoshi, T. Obvious vapochromic color changes of a pillar[6]arene containing one benzoquinone unit with a mechanochromic change before vapor exposure. Chem. Commun. 2020, 56, 4344–4347. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Jie, K.; Zhou, Y.; Zhao, R.; Zhang, B.; Wang, Q.; Liu, J.; Huang, F. Aliphatic Aldehyde Detection and Adsorption by Nonporous Adaptive Pillar[4]arene[1]quinone Crystals with Vapochromic Behavior. Acs Appl. Mater. Interfaces 2018, 10, 23147–23153. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Maruyama, K.; Sakatsume, Y.; Kakuta, T.; Yamagishi, T.-A.; Ichikawa, T.; Mizuno, M. Guest Vapor-Induced State Change of Structural Liquid Pillar[6]arene. J. Am. Chem. Soc. 2019, 141, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, H.; Huang, F. Alkyl Chain Length-Selective Vapor-Induced Fluorochromism of Pillar[5]arene-Based Nonporous Adaptive Crystals. J. Am. Chem. Soc. 2019, 141, 13290–13294. [Google Scholar] [CrossRef]
- Lei, S.-N.; Xiao, H.; Zeng, Y.; Tung, C.-H.; Wu, L.-Z.; Cong, H. BowtieArene: A Dual Macrocycle Exhibiting Stimuli-Responsive Fluorescence. Angew. Chem. Int. Ed. 2020, 59, 10059–10065. [Google Scholar] [CrossRef]
- Ogoshi, T.; Hamada, Y.; Sueto, R.; Kojima, R.; Sakakibara, F.; Nagata, Y.; Sakata, Y.; Akine, S.; Ono, T.; Kakuta, T.; et al. Vapoluminescence Behavior Triggered by Crystal-State Complexation between Host Crystals and Guest Vapors Exhibiting No Visible Fluorescence. Cryst. Growth Des. 2020. [Google Scholar] [CrossRef]
- Li, B.; Cui, L.; Li, C. Macrocycle Co-Crystals Showing Vapochromism to Haloalkanes. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Bishop, R. Designing new lattice inclusion hosts. Chem. Soc. Rev. 1996, 25, 311–319. [Google Scholar] [CrossRef]
- Fei, Z.; Kocher, N.; Mohrschladt, C.J.; Ihmels, H.; Stalke, D. Single Crystals of the Disubstituted Anthracene 9, 10-(Ph2P□S) 2C14H8 Selectively and Reversibly Detect Toluene by Solid-State Fluorescence Emission. Angew. Chem. Int. Ed. 2003, 42, 783–787. [Google Scholar] [CrossRef]
- Ooyama, Y.; Nagano, S.; Okamura, M.; Yoshida, K. Solid-State Fluorescence Changes of 2-(4-Cyanophenyl)-5-[4-(diethylamino)phenyl]-3H-imidazo[4,5-a]naphthalene upon Inclusion of Organic Solvent Molecules. Eur. J. Org. Chem. 2008, 2008, 5899–5906. [Google Scholar] [CrossRef] [Green Version]
- Hinoue, T.; Miyata, M.; Hisaki, I.; Tohnai, N. Guest-Responsive Fluorescence of Inclusion Crystals with π-Stacked Supramolecular Beads. Angew. Chem. Int. Ed. 2012, 51, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Takaya, H.; Naota, T. Dynamic vapochromic behaviors of organic crystals based on the open-close motions of S-shaped donor-acceptor folding units. Chem. Eur. J. 2010, 16, 4793–4802. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Sugimoto, M.; Hisaeda, Y. Multicomponent Molecular Puzzles for Photofunction Design: Emission Color Variation in Lewis Acid–Base Pair Crystals Coupled with Guest-to-Host Charge Transfer Excitation. J. Am. Chem. Soc. 2015, 137, 9519–9522. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, S.; Ono, T.; Hisaeda, Y. Turn-On Fluorogenic and Chromogenic Detection of Small Aromatic Hydrocarbon Vapors by a Porous Supramolecular Host. Chem. Eur. J. 2016, 22, 10346–10350. [Google Scholar] [CrossRef]
- Ono, T.; Tsukiyama, Y.; Taema, A.; Hisaeda, Y. Inclusion Crystal Growth and Optical Properties of Organic Charge-transfer Complexes Built from Small Aromatic Guest Molecules and Naphthalenediimide Derivatives. Chem. Lett. 2017, 46, 801–804. [Google Scholar] [CrossRef] [Green Version]
- Ono, T.; Tsukiyama, Y.; Hatanaka, S.; Sakatsume, Y.; Ogoshi, T.; Hisaeda, Y. Inclusion crystals as vapochromic chemosensors: Fabrication of a mini-sensor array for discrimination of small aromatic molecules based on side-chain engineering of naphthalenediimide derivatives. J. Mater. Chem. C 2019, 7, 9726–9734. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Yang, J.S. Multicolor Fluorescence Writing Based on Host-Guest Interactions and Force-Induced Fluorescence-Color Memory. Angew. Chem. Int. Ed. 2015, 54, 7985–7989. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Maity, S.; Matsunaga, Y.; Hsu, Y.-F.; Liu, Y.-H.; Peng, S.-M.; Shinmyozu, T.; Yang, J.-S. Photomechanochromic vs. mechanochromic fluorescence of a unichromophoric bimodal molecular solid: Multicolour fluorescence patterning. Chem. Sci. 2018, 9, 8990–9001. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-S.; Swager, T.M. Porous Shape Persistent Fluorescent Polymer Films: An Approach to TNT Sensory Materials. J. Am. Chem. Soc. 1998, 120, 5321–5322. [Google Scholar] [CrossRef]
- Yamagishi, H.; Nakajima, S.; Yoo, J.; Okazaki, M.; Takeda, Y.; Minakata, S.; Albrecht, K.; Yamamoto, K.; Badía-Domínguez, I.; Oliva, M.M.; et al. Sigmoidally hydrochromic molecular porous crystal with rotatable dendrons. Commun. Chem. 2020, 3, 118. [Google Scholar] [CrossRef]
- Fujii, K.; Sakon, A.; Sekine, A.; Uekusa, H. Reversible Color Switching of an Organic Crystal Induced by Organic Solvent Vapors. Cryst. Growth Des. 2011, 11, 4305–4308. [Google Scholar] [CrossRef]
- Sakon, A.; Sekine, A.; Uekusa, H. Powder Structure Analysis of Vapochromic Quinolone Antibacterial Agent Crystals. Cryst. Growth Des. 2016, 16, 4635–4645. [Google Scholar] [CrossRef]
- Yano, Y.; Ono, T.; Hatanaka, S.; Gryko, D.T.; Hisaeda, Y. Salt–cocrystal continuum for photofunction modulation: Stimuli-responsive fluorescence color-tuning of pyridine-modified intramolecular charge-transfer dyes and acid complexes. J. Mater. Chem. C 2019, 7, 8847–8854. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, T.; Hisaeda, Y. Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes. Symmetry 2020, 12, 1903. https://doi.org/10.3390/sym12111903
Ono T, Hisaeda Y. Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes. Symmetry. 2020; 12(11):1903. https://doi.org/10.3390/sym12111903
Chicago/Turabian StyleOno, Toshikazu, and Yoshio Hisaeda. 2020. "Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes" Symmetry 12, no. 11: 1903. https://doi.org/10.3390/sym12111903
APA StyleOno, T., & Hisaeda, Y. (2020). Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes. Symmetry, 12(11), 1903. https://doi.org/10.3390/sym12111903