Differential Ageing of the Brain Hemispheres: Evidence from a Longitudinal Study of Hand Preferences in Common Marmosets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Housing
2.2. Scoring Hand Preference
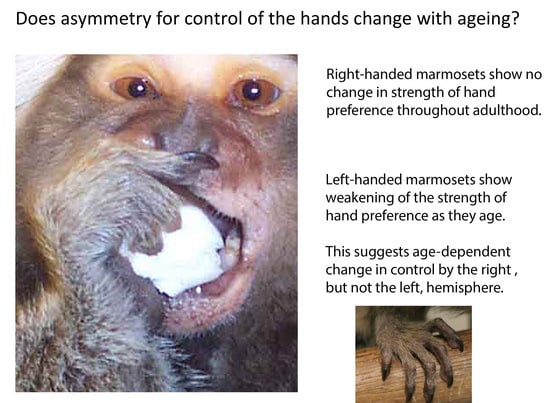
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalisch, T.; Wilimzig, C.; Keibel, N.; Tegenthoff, M.; Dinse, H.R. Age-related attenuation of dominant hand superiority. PLoS ONE 2006, 1, e90. [Google Scholar] [CrossRef]
- Sebastjan, A.; Skrzek, A.; Ignasiak, Z.; Slawińska, T. Age-related changes in hand dominance and functional asymmetry in older adults. PLoS ONE 2017, 12, e0177845. [Google Scholar] [CrossRef] [Green Version]
- Przybyla, A.; Haaland, K.Y.; Bagesteiro, L.B.; Sainburg, R.L. Motor asymmetry reduction in older adults. Neurosci. Lett. 2011, 489, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Raw, R.K.; Wilke, R.M.; Culmer, P.R.; Mon-Williams, M. Reduced motor asymmetry in older adults when manually tracing paths. Exp. Brain Res. 2011, 217, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Przybyla, A.; Wuebbenhorst, K.; Haaland, K.Y.; Sainburg, R.L. Aging reduces asymmetries in interlimb transfer of visuomotor adaptation. Exp. Brain Res. 2011, 210, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Bernard, J.A.; Seidler, R. Hand dominance and age have interactive effects on motor cortical representations. PLoS ONE 2012, 7, e45443. [Google Scholar]
- Talelli, P.; Waddingham, W.; Ewas, A.; Rothwell, J.C.; Ward, N.S. The effect of age on task-related modulation of interhemispheric balance. Exp. Brain Res. 2008, 186, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Talelli, P.; Ewas, A.; Waddingham, W.; Rothwell, J.C.; Ward, N.S. Neural correlates of age-related changes in cortical neurophysiology. NeuroImage 2008, 40, 1772–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellis, T.J.; Nicol, T.; Kraus, N. Aging affects hemispheric asymmetry in the neural representation of speech sounds. J. Neurosci. 2000, 20, 791–797. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.; Deng, Y.; Li, J.; Chen, S.; Wang, C.; Luo, P. Age-associated reduction of asymmetry in human central auditory function: A 1H-magnetic resonance spectroscopy study. Neural Plast. 2013, 2013, 735290. [Google Scholar] [CrossRef] [Green Version]
- Eddins, A.C.; Ozmeral, E.J.; Eddins, D.A. How aging impacts the encoding of binaural cues and the perception of auditory space. Hear. Res. 2018, 369, 79–89. [Google Scholar] [CrossRef]
- Acosta-Sojo, Y.; Martin, B.J. Age-related differences in proprioceptive asymmetries. Neurosci. Lett. 2021, 757, 135992. [Google Scholar] [CrossRef]
- Cabeza, R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol. Aging 2002, 17, 85–100. [Google Scholar] [CrossRef]
- Dolcos, F.; Rice, H.J.; Cabeza, R. Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci. Biobehav. Rev. 2002, 26, 819–825. [Google Scholar] [CrossRef]
- Hill, C.; Van Gemmert, A.W.A.; Fang, Q.; Hou, L.; Wang, J.; Pan, Z. Asymmetry in the aging brain: A narrative review of cortical activation patterns and implications for motor function. Laterality 2020, 25, 413–429. [Google Scholar] [CrossRef]
- Jenkins, L.; Myerson, J.; Joerding, J.A.; Hale, S. Converging evidence that visuospatial cognition is more age-sensitive than verbal cognition. Psychol. Ageing 2000, 15, 157–175. [Google Scholar] [CrossRef]
- Collins, K.; Mohr, C. Performance of younger and older adults in lateralised right and left hemisphere asymmetry tasks supports the HAROLD model. Laterality 2013, 18, 491–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatta, T.; Iwahara, A.; Hatta, T.; Ito, E.; Hatta, J.; Hotta, C.; Nagahara, N.; Fujiwara, K.; Hamajima, N. Developmental trajectories of verbal and visuospatial abilities in healthy older adults: Comparison of the hemisphere asymmetry reduction in older adults model and the right hemi-ageing model. Laterality 2015, 20, 69–81. [Google Scholar] [CrossRef]
- Sugiura, M. Functional neuroimaging of normal aging: Declining brain, adapting brain. Ageing Res. Rev. 2016, 30, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.-W.; Wang, X.-W.; Wang, C.-L.; Zhao, H.-T.; Ren, Y.; Li, B.-G. Effects of age, sex and manual task on hand preference in wild Rhinopithecus roxellana. Zool. Res. 2019, 40, 129–138. [Google Scholar] [PubMed]
- Hook, M.A.; Rogers, L.J. Development of hand preferences in marmosets (Callithrix jacchus) and effects of ageing. J. Comp. Psychol. 2000, 114, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Laurence, A.; Wallez, C.; Blois-Heulin, C. Task complexity, posture, age, sex: Which is the main factor influencing manual laterality in captive Cercocebus torquatus torquatus? Laterality 2011, 16, 586–606. [Google Scholar] [CrossRef]
- Tomassetti, D.; Caracciolo, S.; Manciocco, A.; Chiarotti, F.; Vital, A.; De Filippis, B. Personality and lateralization in common marmosets (Callithrix jacchus). Behav. Processes 2019, 167, 103899. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, E.S.; Freire-Cobo, C.; Varghese, M.; Edwards, M.; Janssen, W.G.M.; Hof, P.R.; Lacreuse, A. The marmoset as an important primate model for longitudinal studies of neurocognitive aging. Am. J. Primatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, E.S.; Workman, K.P.; Wang, D.; Lacreuse, A. Sex differences in cognitive ageing: A 4-year longitudinal study on marmosets. Neurobiol. Aging 2021, 109, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Workman, K.P.; Healey, B.; Carlotto, A.; Lacreuse, A. One-year change in cognitive flexibility and fine motor function in middle-aged male and female marmosets (Callithrix jacchus). Am. J. Primatol. 2019, 81, e22924. [Google Scholar] [CrossRef]
- Geula, C.; Nagykery, N.; Wu, C.K. Amyloid-B deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): Incidence and chemical composition. Acta Neuropathol. 2002, 103, 48–58. [Google Scholar] [CrossRef]
- Clarke, J.M. The common marmoset (Callithrix jacchus). ANZCCAART News 1994, 7, 1–8. [Google Scholar]
- Nishijima, K.; Saitoh, R.; Tanaka, S.; Ohsato-Suzuki, M.; Ohno, T.; Kitajima, S. Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology 2012, 13, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Tardif, S.D.; Mansfield, K.G.; Ratnam, R.; Ross, C.N.; Ziegler, T.E. The marmoset as a model of aging and age-related diseases. ILAR J. 2011, 52, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.N.; Davis, K.; Dobek, G.; Tardif, S.D. Aging phenotypes of common marmosets (Callithrix jacchus). J. Aging Res. 2012, 2012, 567143. [Google Scholar] [CrossRef] [Green Version]
- Nephew, B.C.; Febo, M.; Cali, R.; Workman, K.P.; Payne, L.; Moore, C.M.; King, J.A.; Lacreuse, A. Robustness of sex differences in functional connectivity over time in middle-aged marmosets. Sci. Rep. 2020, 10, 16647. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Rogers, L.J. Differences in social and vocal behavior between left- and right-handed common marmosets. J. Comp. Psychol. 2010, 124, 402–411. [Google Scholar] [CrossRef]
- Pines, M.K.; Kaplan, G.; Rogers, L.J. Use of horizontal and vertical climbing structures by captive common marmosets (Callithrix jacchus). App. Anim. Behav. Sci. 2005, 91, 311–319. [Google Scholar] [CrossRef]
- Hook-Costigan, M.A.; Rogers, L.J. Lateralization of hand, mouth and eye use in the common marmoset (Callithrix jacchus). Folia Primatol. 1995, 64, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Hook, M.A.; Rogers, L.J. Leading-limb preference in marmosets (Callithrix jacchus): Walking, leaping and landing? Laterality 2002, 7, 145–162. [Google Scholar] [CrossRef]
- Cordeiro de Sousa, M.B.; Xavier, N.S.; Alves da Silva, H.P.; Souza de Oliveira, M. Hand preference study in marmosets (Callithrix jacchus) using food reaching tests. Primates 2001, 42, 57–66. [Google Scholar] [CrossRef]
- Hausmann, M.; Güntürkün, O.; Corballis, M.C. Age-related changes in hemispheric asymmetry depend on sex. Laterality 2003, 8, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, L.A. Catergories of manual asymmetry and their variation with advancing age. Cortex 2008, 44, 707–716. [Google Scholar] [CrossRef]
- Davis, S.W.; Kragel, J.E.; Madden, D.J.; Cabeza, R. The architecture of cross-hemispheric communication in the aging brain: Linking behavior to functional and structural connectivity. Cereb. Cortex 2012, 22, 232–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delvenne, J.-F.; Castronovo, J. Reduced interhemispheric interference in ageing: Evidence from a divided field Stroop paradigm. Brain Cogn. 2018, 122, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Pfefferbaum, A.; Adalsteinsson, E.; Swan, G.E.; Carmelli, D. Differential rates of regional brain change in callosal and ventricular size: A 4-year longitudinal MRI study of elderly men. Cereb. Cortex 2002, 12, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Ota, M.; Obata, T.; Akine, Y.; Ito, H.; Ikehira, H.; Asada, T.; Suhara, T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. NeuroImage 2006, 31, 1445–1452. [Google Scholar] [CrossRef]
- Phillips, K.A.; Watson, C.; Bearman, A.; Knippenberg, A.R.; Adams, J.; Ross, C.; Tardif, S.D. Age-related changes in myelin of axons of the corpus callosum and cognitive decline in common marmosets. Am. J. Primatol. 2019, 81, e22949. [Google Scholar] [CrossRef]
- Manns, M.; Otto, T.; Salm, L. Pigeons show how meta-control enables decision-making in an ambiguous world. Sci. Rep. 2021, 11, 3838. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Güntürkün, O. The commissura anterior compensates asymmetries of visual representation in pigeons. Laterality 2021, 26, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, G.; Benwell, C.S.Y.; Thut, G.; Harvey, M. Age-related reduction of hemispheric lateralisation for spatial attention: An EEG study. NeuroImage 2017, 153, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hakun, J.G.; Johnson, N.F.; Gold, B.T. Age-related increases in right frontal activation during task switching are mediated by reaction time in white matter microstructure. Neurosci. 2014, 278, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Bureš, Z.; Pysanenko, K.; Syka, J. Age-related changes in the temporal processing of acoustic signals in the auditory cortex of rats. Hear. Res. 2021, 402, 108025. [Google Scholar] [CrossRef]
- Goldstein, G.; Shelly, C. Does the right hemisphere age more rapidly than the left? J. Clin. Neuropsychol. 1981, 3, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Gorrie, C.A.; Waite, P.M.E.; Rogers, L.J. Correlations between hand preference and cortical thickness in the secondary somatosensory (SII) cortex. Behav. Neurosci. 2008, 122, 1343–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, E.; Le, A.; Casey, M.; Workman, K.P.; Lacreuse, A. Baseline cortisol levels and social behavior differ as a function of handedness in marmosets (Callithrix jacchus). Am. J. Primatol. 2019, 81, e23057. [Google Scholar] [CrossRef]
- Gordon, D.J.; Rogers, L.J. Cognitive bias, hand preference and welfare in common marmosets. Behav. Brain Res. 2015, 287, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Zach, P.; Vales, K.; Stuchlík, A.; Čermáková, P.; Mrzílková, J.; Koutella, A.; Kutová, M. Effect of stress on structural brain asymmetry. Neuroendocrinol. Lett. 2016, 37, 101–112. [Google Scholar]
- Zach, P.; Mrzílková, J.; Rezacova, L.; Stuchlík, A.; Vales, K. Delayed effects of elevated corticosterone level on volume of hippocampal formation in laboratory rat. Physiol. Res. 2010, 59, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Frodl, T.; O’Keane, V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 2013, 52, 24–37. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Landfield, P.W.; Blalock, E.M.; Chen, K.C.; Porter, N.M. A new glucocorticoid hypothesis of brain aging: Implications for Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 205–212. [Google Scholar] [CrossRef]
- Pereira, L.C.; Maior, R.S.; Barros, M. Time-dependent changes in cortisol and tympanic temperature lateralization during food deprivation stress in marmoset monkeys. Front. Behav. Neurosci. 2020, 14, 123. [Google Scholar] [CrossRef]
- Sivagnanasunderam, M.; Gonzalez, D.A.; Bryden, P.J.; Young, G.; Forsyth, A.; Roy, E.A. Handedness throughout the lifespan: Cross-sectional view on sex differences as asymmetries change. Front. Psychol. 2015, 5, 1556. [Google Scholar] [CrossRef] [Green Version]
- Hook-Costigan, M.A.; Rogers, L.J. Eye preferences in common marmosets (Callithrix jacchus): Influence of age, stimulus, and hand preference. Laterality 1998, 3, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.M.; Vidal-Piñeiro, D.; Sørensen, Ø.; Brandmaier, A.M.; Düzel, S.; Gonzalez, H.A.; Kievit, R.A.; Knights, E.; Kühn, S.; Lindenberger, U.; et al. Asymmetric thinning of the cerebral cortex across the adult lifespan is accelerated in Alzheimer’s disease. Nat. Comm. 2021, 12, 721. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, L.J. Differential Ageing of the Brain Hemispheres: Evidence from a Longitudinal Study of Hand Preferences in Common Marmosets. Symmetry 2021, 13, 2349. https://doi.org/10.3390/sym13122349
Rogers LJ. Differential Ageing of the Brain Hemispheres: Evidence from a Longitudinal Study of Hand Preferences in Common Marmosets. Symmetry. 2021; 13(12):2349. https://doi.org/10.3390/sym13122349
Chicago/Turabian StyleRogers, Lesley J. 2021. "Differential Ageing of the Brain Hemispheres: Evidence from a Longitudinal Study of Hand Preferences in Common Marmosets" Symmetry 13, no. 12: 2349. https://doi.org/10.3390/sym13122349
APA StyleRogers, L. J. (2021). Differential Ageing of the Brain Hemispheres: Evidence from a Longitudinal Study of Hand Preferences in Common Marmosets. Symmetry, 13(12), 2349. https://doi.org/10.3390/sym13122349