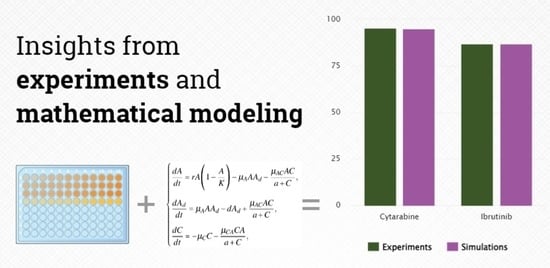
Differential Response to Cytotoxic Drugs Explains the Dynamics of Leukemic Cell Death: Insights from Experiments and Mathematical Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation of the Model
2.2. Estimation of the Parameters
- (cells/mL)-the initial number of A20 mCherry cells;
- (cells/mL)-the initial number of dead A20 mCherry cells (cell cultures commonly consists of at least 5% of dead cells);
- dose (M) (number of drug molecules/mL)-the dose concentration of Ibr or Cyt (this number may vary depending on the drug, but not significantly since both drugs are related to the same type of small molecules).
- m = the mass of drug in kg,
- = avogadro number = (constant),
- M = the molar mass of drug (Ibr 440.5 g/mol; Cyt 243.217 g/mol).
2.3. Cells and Reagents
2.4. Drug Cytotoxicity Assay
3. Results
- To simulate the impact of Cyt and Ibr drugs on killing A20 leukemic cells in silico;
- To predict with a high level of accuracy of the cytotoxic efficacy of Cyt and Ibr drugs for high doses in comparison with the results in vitro experiments.
3.1. Validation of the In Vitro A20 mCherry Cell Drug Cytotoxicity Dynamic Model
3.2. Goodness of Fitting Evolution
3.3. Prediction of the Synergistic Effect of Drugs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Simulated Effect of Drugs on A20 Cells
| Concentration of Ibr (µM) | Parameter | Number of Cells at 72nd Hour (Cells/mL) | Cell Growth Inhibition (%) |
|---|---|---|---|
| 0 | - | 2,636,950 | 0 |
| 50 | 0.066 | 50,953 | 98.1 |
| 25 | 0.046 | 229,415 | 91.3 |
| 0.032 | 424,548 | 83.9 | |
| 0.022 | 743,620 | 71.8 | |
| 0.015 | 1,152,347 | 56.3 | |
| 0.01 | 1,579,533 | 40.1 | |
| 0.007 | 1,914,425 | 27.4 | |
| 0.005 | 2,244,044 | 14.9 | |
| 0.003 | 2,539,382 | 3.7 |
| Concentration of Cyt (µM) | Parameter | Number of Cells at 72nd Hour (Cells/mL) | Cell Growth Inhibition (%) |
|---|---|---|---|
| 0 | - | 2,636,950 | 0 |
| 0.051 | 137,121 | 94.8 | |
| 0.036 | 268,969 | 89.8 | |
| 0.025 | 466,740 | 82.3 | |
| 0.017 | 725,161 | 72.5 | |
| 0.012 | 1,020,500 | 61.3 | |
| 0.008 | 1,326,386 | 49.7 | |
| 0.006 | 1,608,540 | 39 | |
| 0.004 | 1,853,776 | 29.7 | |
| 0.003 | 2,051,547 | 22.2 | |
| 0.002 | 2,209,764 | 16.2 | |
| 0.0014 | 2,328,427 | 11.7 |
Appendix B
The Finite-Distance Analysis Error of the Model
References
- Kay, N.E.; Hampel, P.J.; Van Dyke, D.L.; Parikh, S.A. CLL update 2022: A continuing evolution in care. Blood Rev. 2022, 54, 100930. [Google Scholar] [CrossRef] [PubMed]
- Bewarder, M.; Stilgenbauer, S.; Thurner, L.; Kaddu-Mulindwa, D. Current Treatment Options in CLL. Cancers 2021, 13, 2468. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, N.; Gandhi, V. Ibrutinib combinations in CLL therapy: Scientific rationale and clinical results. Blood Cancer J. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thimiri, G.R.; Deepak, B.b.; Cremaschi, A.; Skanland, S.S.; Gade, A.; Schjesvold, F.H.; Tjonnfjord, G.E.; A Munthe, L.; Tasken, K. In-vitro drug sensitivity screening in chronic lymphocytic leukemia (CLL) primary patient samples identifies drug candidates for precision cancer therapy. Blood 2018, 132, 4676. [Google Scholar] [CrossRef]
- Lukas, M.; Velten, B.; Sellner, L.; Tomska, K.; Hüellein, J.; Walther, T.; Wagner, L.; Muley, C.; Wu, B.; Oleś, M.; et al. Survey of ex vivo drug combination effects in chronic lymphocytic leukemia reveals synergistic drug effects and genetic dependencies. Leukemia 2020, 34, 2934–2950. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rai, K.R. Chronic lymphocytic leukemia (CLL) treatment: So many choices, such great options. Cancer 2019, 125, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Bunimovich-Mendrazitsky, S.; Kronik, N.; Vainstein, V. Optimization of Interferon–Alpha and Imatinib Combination Therapy for Chronic Myeloid Leukemia: A Modeling Approach. Adv. Theory Simul. 2019, 2, 1800081. [Google Scholar] [CrossRef]
- Jost, F.; Schalk, E.; Rinke, K.; Fischer, T.; Sager, S. Mathematical models for cytarabine-derived myelosuppression in acute myeloid leukaemia. PLoS ONE 2019, 14, e0204540. [Google Scholar] [CrossRef] [PubMed]
- Clapp, G.; Levy, D. A review of mathematical models for leukemia and lymphoma. Drug Discov. Today Dis. Model. 2015, 16, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhury, A.; Zhu, X.; Chu, L.; Goliaei, A.; June, C.H.; Kearns, J.D.; Stein, A.M. Chimeric Antigen Receptor T Cell Therapies: A Review of Cellular Kinetic-Pharmacodynamic Modeling Approaches. J. Clin. Pharmacol. 2020, 60, S147–S159. [Google Scholar] [CrossRef] [PubMed]
- Rea, T.; Guillari, A.; Sergi, C.; Serra, N. Mathematical models in nursing research. J. Public Health Res. 2020, 9, jphr.2020.1952. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.S.; Mancera, P.F.; Carvalho, T.; Gonçalves, L.F. A mathematical model for chemoimmunotherapy of chronic lymphocytic leukemia. Appl. Math. Comput. 2019, 349, 118–133. [Google Scholar] [CrossRef] [Green Version]
- Benzekry, S.; Lamont, C.; Beheshti, A.; Tracz, A.; Ebos, J.M.; Hlatky, L.; Hahnfeldt, P. Classical mathematical models for description and prediction of experimental tumor growth. PLoS Comput. Biol. 2014, 10, e1003800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzev, E.; Luboshits, G.; Bunimovich-Mendrazitsky, S.; Firer, M.A. Experimental Validation of a Mathematical Model to Describe the Drug Cytotoxicity of Leukemic Cells. Symmetry 2021, 13, 1760. [Google Scholar] [CrossRef]
- Faruqi, A.; Tadi, P. Cytarabine. Available online: http://europepmc.org/books/NBK557680 (accessed on 17 June 2022).
- Ganatra, S.; Sharma, A.; Shah, S.; Chaudhry, G.M.; Martin, D.T.; Neilan, T.G.; Mahmood, S.S.; Barac, A.; Groarke, J.D.; Hayek, S.S.; et al. Ibrutinib-associated atrial fibrillation. JACC Clin. Electrophysiol. 2018, 4, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Komarova, N.L.; Burger, J.A.; Wodarz, D. Evolution of ibrutinib resistance in chronic lymphocytic leukemia (CLL). Proc. Natl. Acad. Sci. USA 2014, 111, 13906–13911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Hu, P.; Yan, X.; Zhang, Y.; Shi, W. Ibrutinib in chronic lymphocytic leukemia: Clinical applications, drug resistance, and prospects. Oncotargets Ther. 2020, 13, 4877. [Google Scholar] [CrossRef] [PubMed]
- Aroesty, J.; Lincoln, T.; Shapiro, N.; Boccia, G. Tumor growth and chemotherapy: Mathematical methods, computer simulations, and experimental foundations. Math. Biosci. 1973, 17, 243–300. [Google Scholar] [CrossRef]
- Bellman, R. Mathematical Methods in Medicine; World Scientific: Singapore, 1983; Volume 1. [Google Scholar]
- Lazebnik, T.; Aaroni, N.; Bunimovich-Mendrazitsky, S. PDE based geometry model for BCG immunotherapy of bladder cancer. Biosystems 2021, 200, 104319. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.D.; Hindmarsh, A.C. Stiff ODE solvers: A review of current and coming attractions. J. Comput. Phys. 1987, 70, 1–62. [Google Scholar] [CrossRef]




| Parameter | Physical Interpretation (Units) | Estimated Value | Reference |
|---|---|---|---|
| The initial number of A20 [cells/mL] | Experimental data | ||
| The initial number of dead A20 [cells/mL] | 2500 | Experimental data | |
| Number of drug [molecules/mL] | dose (M) | Experimental data | |
| t | Time of cell culture [h] | 0–72 | Experimental data |
| r | A20 growth rate [h] | 0.07 | Experimental data |
| K | Maximal tumor cell population [cells/mL] | Experimental data | |
| Living cells become dead [h] | Simulation | ||
| a | Drug dose that produces 50% maximum effect [mL] | [12] | |
| Cytotoxicity rate in the presence of drug [h] | see Table A1 and Table A2 | Simulation | |
| Deactivation rate of drug due to killing of A20 cells [h] | Simulation | ||
| Chemical deactivation rate of drug [h] | 0.231-Cyt; 0.116-Ibr | [12] | |
| d | Dissolution rate of dead A20 cells [h] | 0.017 | Simulation |
| Concentration of Drug (µM) | Cytarabine | Ibrutinib | ||
|---|---|---|---|---|
| Exp | Sim | Exp | Sim | |
| 50 | 97.5 | 98.1 | ||
| 25 | 91.5 | 91.3 | ||
| 12.5 | 74 | 83.9 | ||
| 6.25 | 95.2 | 94.8 | 42.5 | 71.8 |
| 3.125 | 93.5 | 89.8 | 29.1 | 56.3 |
| 1.5625 | 88.7 | 82.3 | 20.3 | 40.1 |
| 0.78 | 87 | 72.5 | 18.8 | 27.4 |
| 0.39 | 85.2 | 61.3 | 10.8 | 14.9 |
| 0.195 | 80.5 | 49.7 | 4.3 | 3.7 |
| 0.098 | 66.25 | 39 | ||
| 0.049 | 29.5 | 29.7 | ||
| 0.024 | 18.9 | 22.2 | ||
| 0.012 | 14.5 | 16.2 | ||
| 0.006 | 7.7 | 11.7 | ||
| RMSE | 0.018 | 0.032 | ||
| MAPE | 0.198 | 0.412 | ||
| Concentration of Drugs (µM) | Parameter | Number of Cells at 72nd Hour (Cells/mL) | Cell Growth Inhibition (%) |
|---|---|---|---|
| 0 | - | 2,636,950 | 0 |
| Ibr 3.125 | 0.015 | 1,152,347 | 56.3 |
| Cyt 0.195 | 0.008 | 1,326,386 | 49.7 |
| Ibr 3.125 + Cyt 0.195 | 0.023 | 702,330 | 73.4 |
| Ibr 6.25 | 0.022 | 743,620 | 71.8 |
| Cyt 0.39 | 0.012 | 1,020,500 | 61.3 |
| Ibr 6.25 + Cyt 0.39 | 0.034 | 375,766 | 85.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzev, E.; Bunimovich-Mendrazitsky, S.; Firer, M.A. Differential Response to Cytotoxic Drugs Explains the Dynamics of Leukemic Cell Death: Insights from Experiments and Mathematical Modeling. Symmetry 2022, 14, 1269. https://doi.org/10.3390/sym14061269
Guzev E, Bunimovich-Mendrazitsky S, Firer MA. Differential Response to Cytotoxic Drugs Explains the Dynamics of Leukemic Cell Death: Insights from Experiments and Mathematical Modeling. Symmetry. 2022; 14(6):1269. https://doi.org/10.3390/sym14061269
Chicago/Turabian StyleGuzev, Ekaterina, Svetlana Bunimovich-Mendrazitsky, and Michael A. Firer. 2022. "Differential Response to Cytotoxic Drugs Explains the Dynamics of Leukemic Cell Death: Insights from Experiments and Mathematical Modeling" Symmetry 14, no. 6: 1269. https://doi.org/10.3390/sym14061269
APA StyleGuzev, E., Bunimovich-Mendrazitsky, S., & Firer, M. A. (2022). Differential Response to Cytotoxic Drugs Explains the Dynamics of Leukemic Cell Death: Insights from Experiments and Mathematical Modeling. Symmetry, 14(6), 1269. https://doi.org/10.3390/sym14061269