Relating Fluctuating Asymmetries and Mean Values and Discordances of Asymmetries in a Set of Morphological Traits
Abstract
:1. Introduction
2. Relationships between FAs and Trait Means in a Set of Multiple Traits
3. Differences in the Regression of Slope of FAs—Means in Natural Populations
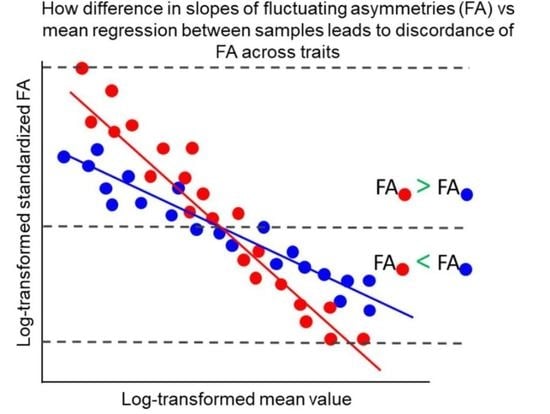
4. Discordance of FAs across Traits and Measuring Developmental Instability
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry: Measurement, analysis, patterns. Ann. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Zakharov, V.M. Future prospects for population phenogenetics. Sov. Sci. Rev. Sect. F Physiol. Gen. Bio. Rev. 1989, 4, 1–79. [Google Scholar]
- Parsons, P.A. Fluctuating asymmetry: An epigenetic measure of stress. Biol. Rev. 1990, 65, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Emlen, J.M.; Freeman, D.C. Developmental stability and its applications in ecotoxicology. Ecotoxicology 1993, 2, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lajus, D.L.; Golovin, P.V.; Yurtseva, A.O.; Ivanova, T.S.; Dorgham, A.S.; Ivanov, M.V. Fluctuating asymmetry as an indicator of stress and fitness in stickleback: Analysis of publications and testing cranial structures. Evol. Ecol. Res. 2019, 20, 83–106. Available online: http://evolutionary-ecology.com/abstracts/v20/3165.html (accessed on 12 December 2022).
- Polak, M. (Ed.) Developmental Instability: Causes and Consequences; Oxford University Press: New York, NY, USA; Oxford, UK, 2003. [Google Scholar]
- Lajus, D. God playing dice, revisited: Determinism and indeterminism in studies of stochastic phenotypic variation. Emerg. Top. Life Sci. 2022, 6, 303–310. [Google Scholar] [CrossRef]
- Van Dongen, S.V. Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. J. Evol. Biol. 2006, 19, 1727–1743. [Google Scholar] [CrossRef]
- Graham, J.H.; Raz, S.; Hel-Or, H.; Nevo, E. Fluctuating asymmetry: Methods, theory, and applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef]
- Allenbach, D.M. Fluctuating asymmetry and exogenous stress in fishes: A review. Rev. Fish Biol. Fish. 2011, 21, 355–376. [Google Scholar] [CrossRef]
- Graham, J.H. Fluctuating asymmetry and developmental instability, a guide to best practice. Symmetry 2021, 13, 9. [Google Scholar] [CrossRef]
- Graham, J.H. Nature, nurture, and noise: Developmental instability, fluctuating asymmetry, and the causes of phenotypic variation. Symmetry 2021, 13, 1204. [Google Scholar] [CrossRef]
- Dufour, K.M.; Weatherhed, P.J. Estimation of organism-wide asymmetry in red-winged blackbirds and its relation to studies of mail selection. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1996, 263, 769–775. [Google Scholar]
- Clarke, G.M. The genetic basis of developmental stability: IV. Individual and population asymmetry parameters. Heredity 1998, 80, 553–561. [Google Scholar] [CrossRef]
- Zakharov, V.M. Linking developmental stability and environmental stress: A whole organism approach. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2003; pp. 402–414. [Google Scholar]
- Leung, B.; Forbes, M.R. Fluctuating asymmetry in relation to stress and fitness: Effects of trait type as revealed by meta-analysis. Ecoscience 1996, 3, 400–413. [Google Scholar] [CrossRef]
- Bjorksten, T.A.; Fowler, K.; Pomiankowski, A. What does sexual trait FA tell us about stress? Tr. Ecol. Evol. 2000, 15, 163–166. [Google Scholar] [CrossRef]
- Soulé, M.E. Phenetics of natural populations. II: Asymmetry and evolution in a lizard. Amer. Nat. 1967, 101, 141–160. [Google Scholar] [CrossRef]
- Soulé, M.E.; Baker, B. Phenetics of natural populations. IV. The population asymmetry parameter in the butterfly. Coenonym phatullia. Heredity 1968, 23, 611–614. [Google Scholar] [CrossRef]
- Jagoe, C.H.; Haines, T.A. Fluctuating asymmetry in fishes inhabiting acidified and unacidified lakes. Can. J. Zool. 1985, 63, 130–138. [Google Scholar] [CrossRef]
- Zhivotovsky, L.A. A measure of fluctuating asymmetry for a set of characters. Acta Zool. Fenn. 1992, 191, 73–77. [Google Scholar]
- Whitlock, M. Lack of correlation between heterozigosity and fitness in forked fungus beetles. Heredity 1993, 70, 571–584. [Google Scholar] [CrossRef]
- Palmer, A.R. Fluctuating asymmetry analyses: A primer. In Developmental Stability: Its Origin and Evolutionary Implications; Markow, T.A., Ed.; Kluwer Academic Press: Dortrecht, The Netherlands, 1994; pp. 335–364. [Google Scholar]
- Bennett, D.M.; Hoffmann, A.A. Effects of size and fluctuating asymmetry on field fitness of the parasitoid Trichogramm carverae (Hymenoptera: Trichogrammatidae). J. Anim. Ecol. 1998, 64, 580–591. [Google Scholar] [CrossRef]
- Leung, B.; Forbes, M.R.; Houle, D. Fluctuating asymmetry as a bioindicator of stress: Comparing efficacy of analyses involving multiple traits. Amer. Nat. 2000, 155, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry analyses revisited. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2003; pp. 279–319. [Google Scholar]
- Auffray, J.-C.; Alibert, P.; Renaud, S.; Orth, A.; Bonhomme, F. Fluctuating asymmetry in Mus musculus subspecific hybridization. In Traditional and Procrustes Comparative Approach. In Advances in Morphometrics; Marcus, L.F., Corti, M., Loy, A., Naylor, G.J.P., Slice, D.E., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 275–283. [Google Scholar]
- Klingenberg, C.P. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Shape asymmetry—what’s new? Emerg. Top. Life. Sci. 2022, 6, 285–294. [Google Scholar] [CrossRef]
- Møller, A.P.; Swaddle, J.P. Asymmetry, Developmental Stability and Evolution; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Kozhara, A.V. Phenotypic variance of bilateral characters as an indicator of genetic and environmental conditions in bream Abramis brama (L.) (Pisces, Cyprinidae) population. J. Appl. Ichth. 1994, 10, 167–181. [Google Scholar] [CrossRef]
- Leamy, L. Morphometric studies in inbred and hybrid house mice. VII. Heterosis in fluctuating asymmetry at different ages. Acta Zool. Fenn. 1992, 191, 111–119. [Google Scholar]
- Leary, R.F.; Allendorf, F.W.; Knudsen, K.L. Genetic, environmental, and developmental causes of meristic variation in rainbow trout. Acta Zool. Fenn. 1992, 191, 79–95. [Google Scholar]
- Auffray, J.-C.; Debat, V.; Alibert, P. Shape asymmetry and developmental stability. In On Growth and Form: Spatio-Temporal Pattern Formation in Biology; Chaplain, M.A.J., Singh, G.D., McLachlan, J.C., Eds.; John Wiley and Sons Ltd.: Chichester, UK, 1999; pp. 309–324. [Google Scholar]
- Lajus, D.; Yurtseva, A.; Birch, G.; Booth, D. Fluctuating asymmetry as a pollution monitor: The Australian estuarine smooth toadfish Tetractenos glaber (Teleostei: Tetraodontidae). Mar. Poll. Bull. 2015, 101, 758–767. [Google Scholar] [CrossRef]
- Lajus, D.; Katolikova, M.; Strelkov, P.; Hummel, H. Fluctuating and directional asymmetry of the blue mussel (Mytilus edulis): Improving methods of morphological analysis to explore species performance at the northern border of its range. Symmetry 2015, 7, 488–514. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Woods, R.E. Associating stress with developmental stability: Problems and patterns. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2003; pp. 387–401. [Google Scholar]
- Freeman, D.C.; Brown, M.L.; Duda, J.J.; Graham, J.H.; Emlen, J.M.; Krzysik, A.J.; Balbach, H.; Kovacic, D.A.; Zak, J.C. Leaf fluctuating asymmetry, soil disturbance and plant stress: A multiple year comparison using two herbs, Ipomoea pandurate and Cnidoscolus stimulosus. Ecol. Indic. 2005, 5, 85–95. [Google Scholar] [CrossRef]
- Leung, B.; Forbes, M.R. Modelling fluctuating asymmetry in relation to stress and fitness. Oikos 1997, 78, 397–405. [Google Scholar] [CrossRef]
- Lens, L.; Van Dongen, S.; Kark, S.; Matthysen, E. Fluctuating asymmetry as an indicator of fitness: Can we bridge the gap between studies? Biol. Rev. 2002, 77, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. A developmental perspective on developmental instability: Theory, models, and mechanisms. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2003; pp. 14–34. [Google Scholar]
- Graham, J.H.; Shimizu, K.; Emlen, J.M.; Freeman, D.C.; Merkel, J. Growth models and expected distribution of fluctuating asymmetry. Biol. J. Linn. Soc. 2003, 80, 57–65. [Google Scholar] [CrossRef]
- Pearson, K.; Davin, A.G. On the biometric constants of the human skull. Biometrica 1924, 16, 328–363. [Google Scholar] [CrossRef]
- Huestis, R.R. A description of microscopic hair characters and of their inheritance in Peromyscus. J. Exp. Zool. 1925, 41, 429–470. [Google Scholar] [CrossRef]
- Roginskii, Y.Y. On some results of the use of quantitative method for morphological variation studies. Arkhiv Anat. Gistol. I Embriol. 1959, 36, 83–89. (In Russian) [Google Scholar]
- Kurshakova, Y.S. On the classification of measurable qualitative characters. Zhurn. Obs. Biol. 1967, 28, 306–314. (In Russian) [Google Scholar]
- Yegorov, J.E. Range of variability and its connection with character’s absolute mean values and processes of formation. Zhurnal Obs. Biol. 1969, 30, 658–662. (In Russian) [Google Scholar]
- Yablokov, A.V. Variability of Mammals; Amerind Publishing Co: New Delhi, India, 1974. [Google Scholar]
- Lande, R. On comparing coefficients of variation. Syst. Zool. 1977, 26, 214–217. [Google Scholar] [CrossRef]
- Soulé, M.E. Allomeric variation. I. The theory and some consequences. Amer. Nat. 1982, 120, 751–764. [Google Scholar] [CrossRef]
- Leamy, L. Morphometric studies in inbred and hybrid house mice. V. Directional and fluctuating asymmetry. Amer. Nat. 1984, 123, 579–593. [Google Scholar] [CrossRef]
- Lajus, D.L. Variation patterns of bilateral characters: Variation among characters and among populations in the White Sea herring (Clupea pallasi marisalbi). Biol. J. Linn. Soc. 2001, 74, 237–253. [Google Scholar]
- Lajus, D.L.; Alekseev, V.R. Components of morphological variation in baikalian endemial cyclopid Acanthocyclops signifer complex from different localities. Hydrobiologia 2000, 417, 25–35. [Google Scholar] [CrossRef]
- Lajus, D.L.; Knust, R.; Brix, O. Fluctuating asymmetry and other parameters of morphological variation of eelpout Zoarces viviparus from different parts of distributional range. Sarsia 2003, 88, 247–260. [Google Scholar] [CrossRef]
- Lajus, D.L.; Ciostek, M.; Draszanowska, M.; Sywula, T. Geographic and ontogenetic patterns of chaetotaxy variation in glacial relict Saduria entomon (L.) (Crustacea, Isopoda): Inter-population, inter-individual and intra-individual variation (fluctuating asymmetry). Ann. Zool. Fenn. 2003, 40, 411–429. [Google Scholar]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Lajus, D.L.; Graham, J.H.; Kozhara, A.V. Developmental instability and the stochastic component of total phenotypic variance. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2003; pp. 343–363. [Google Scholar]
- Mosimann, J.E.; Campbell, G. Applications in biology: Simple growth models. In Lognormal distributions: Theory and applications; Crow, E.L., Shimizu, K., Eds.; Marcel Dekker: New York, NY, USA, 1988; pp. 287–302. [Google Scholar]
- Graham, J.H.; Emlen, J.M.; Freeman, D.C. Nonlinear dynamics and developmental instability. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2003; pp. 35–50. [Google Scholar]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry as a measure of developmental stability: Implications of nonnormal distributions and power of statistical tests. Acta Zool. Fenn. 1992, 191, 57–72. [Google Scholar]
- Emlen, J.M.; Freeman, C.D.; Graham, J.H. Nonlinear growth dynamics and origin of fluctuating asymmetry. Genetica 1993, 89, 77–96. [Google Scholar] [CrossRef]
- Graham, J.H.; Freeman, D.C.; Emlen, J.M. Antisymmetry, directional symmetry, and dynamic morphogenesis. Genetica 1993, 89, 121–137. [Google Scholar] [CrossRef]
- Van Dongen, S.; Sprengers, E.; Löfstedt, C. Correlated development, organism wide asymmetry and patterns of asymmetry in two moth species. Genetica 1999, 105, 81–91. [Google Scholar] [CrossRef]
- Freeman, C.D.; Brown, M.L.; Dobson, M.; Jordan, Y.; Kizi, A.; Micallef, C.; Hancock, L.C.; Emlen, J.M.; Graham, J.H. 2003. Developmental instability: Measures of resistance and resilience using pampkin (Cucurbito pepo L.). Biol. J. Linn. Soc. 2003, 78, 27–41. [Google Scholar] [CrossRef]
- Vogt, G. Stochastic developmental variation, an epigenetic source of phenotypic diversity with far-reaching biological consequences. J. Biosci. 2015, 40, 159–204. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, F.J. Biometry; W.H. Freeman and Company: New York, NY, USA, 1981. [Google Scholar]
- Lajus, D.L. What is the White Sea herring (Clupea pallasi marisalbi, Berg 1923)? A new concept of the population structure. In Proceedings of the VIII Congress Societatis Europaea Ichtiologorum, Oviedo, Spain, 26 September–2 October 1996; Volume 21, pp. 221–230. [Google Scholar]
- Lajus, D.L. Two-dimensional model of phenotypic variation. Proc. Zool. Inst. RAS 1998, 267, 107–114. (In Russian) [Google Scholar]

| Species, Populations and Trait Type | Pearson Correlation Coefficient | Slope FA-M, Log-Transformed | Slope FC-M, Log-Transformed | ||
|---|---|---|---|---|---|
| Untransformed FA and M | Square-Root Transformed M | Log-Transformed M and FA | |||
| A. signifer (1), metric | −0.367 | −0.522 | −0.499 | −0.511 | −0.141 |
| A. signifer (2), metric | −0.461 | −0.570 | −0.684 | −1.325 | −0.547 |
| A. signifer (3), metric | −0.395 | −0.562 | −0.908 | −1.192 | −0.217 |
| C. pallasii (1), meristic | −0.550 | −0.660 | −0.799 | −0.862 | −0.637 |
| C. pallasii (2), meristic | −0.587 | −0.702 | −0.954 | −1.276 | −0.493 |
| C. pallasii (1), metric | −0.598 | −0.688 | −0.802 | −1.883 | −1.451 |
| C. pallasii (2), metric | −0.646 | −0.735 | −0.853 | −2.048 | −1.546 |
| Z. viviparus (1), meristic | −0.672 | −0.724 | −0.831 | −0.742 | −0.569 |
| Z. viviparus (2), meristic | −0.471 | −0.558 | −0.826 | −0.916 | −0.563 |
| Z. viviparus (3), meristic | −0.551 | −0.652 | −0.847 | −0.886 | −0.477 |
| Z. viviparus (4), meristic | −0.646 | −0.769 | −0.813 | −0.872 | −0.113 |
| Z. viviparus (5), meristic | −0.546 | −0.634 | −0.847 | −0.740 | −0.042 |
| S. entomon (1), meristic | −0.482 | −0.506 | −0.454 | −0.934 | −1.736 |
| S. entomon (2), meristic | −0.736 | −0.767 | −0.763 | −1.125 | −0.481 |
| S. entomon (3), meristic | −0.459 | −0.477 | −0.491 | −1.142 | −0.466 |
| S. entomon (4), meristic | −0.268 | −0.250 | −0.229 | −0.355 | 0.040 |
| S. entomon (5), meristic | −0.581 | −0.612 | −0.628 | −1.083 | 1.491 |
| S. entomon (6), meristic | −0.773 | −0.782 | −0.829 | −1.222 | −0.383 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajus, D.L. Relating Fluctuating Asymmetries and Mean Values and Discordances of Asymmetries in a Set of Morphological Traits. Symmetry 2023, 15, 476. https://doi.org/10.3390/sym15020476
Lajus DL. Relating Fluctuating Asymmetries and Mean Values and Discordances of Asymmetries in a Set of Morphological Traits. Symmetry. 2023; 15(2):476. https://doi.org/10.3390/sym15020476
Chicago/Turabian StyleLajus, Dmitry L. 2023. "Relating Fluctuating Asymmetries and Mean Values and Discordances of Asymmetries in a Set of Morphological Traits" Symmetry 15, no. 2: 476. https://doi.org/10.3390/sym15020476
APA StyleLajus, D. L. (2023). Relating Fluctuating Asymmetries and Mean Values and Discordances of Asymmetries in a Set of Morphological Traits. Symmetry, 15(2), 476. https://doi.org/10.3390/sym15020476