Power Generation: Feedstock for High-Value Sulfate Minerals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Industrial Effluent
2.2. Barite Recovery
2.3. Point of Zero Charge (PZC)
2.4. Toxicity Characteristic Leaching Procedure (TCLP)
2.5. Density Determination of Barite
2.6. Analytics
3. Results
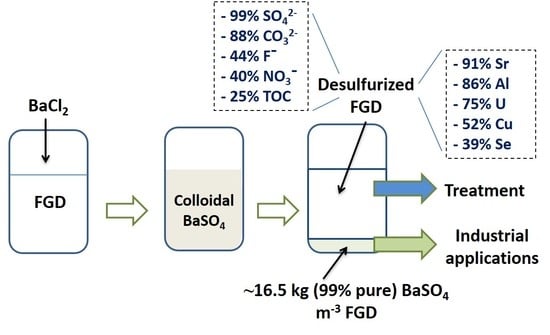
3.1. FGD Characterization
3.2. FGD Desulfurization and BaSO4 Formation
3.3. Characterization of BaSO4
3.4. BaSO4 Stability
4. Discussion
4.1. Desulfurization of the FGD Effluent
4.2. Barite Characterization
4.3. Reuse of BaSO4 and Preliminary Cost-Benefit Analysis of the Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- British Petroleum (BP) (2018) Statistical Review of World Energy. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2018-full-report.pdf (accessed on 15 September 2019).
- Eurocoal Statistics. Available online: https://euracoal.eu/info/euracoal-eu-statistics/ (accessed on 17 February 2020).
- Energy Topics. Available online: https://ec.europa.eu/energy/en/topics/oil-gas-and-coal/coal-and-other-solid-fuels (accessed on 10 October 2019).
- International Energy Agency. Available online: https://www.iea.org/countries/poland (accessed on 17 February 2020).
- Clean Energy Wire: Coal in Germany. Available online: https://www.cleanenergywire.org/factsheets/coal-germany (accessed on 10 October 2019).
- Cordoba, P. Partitioning and speciation of selenium in wet limestone flue gas desulphurisation (FGD) systems: A review. Fuel 2017, 202, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Gingerich, D.B.; Grol, E.; Mauter, M.S. Fundamental challenges and engineering opportunities in flue gas desulfurization wastewater treatment at coal fired power plants. Environ. Sci. Water Res. Technol. 2018, 4, 909–925. [Google Scholar] [CrossRef]
- Heidel, B.; Rogge, T.; Scheffknecht, G. Controlled desorption of mercury in wet FGD waste water treatment. Appl. Energy 2016, 162, 1211–1217. [Google Scholar] [CrossRef]
- Staicu, L.C.; Morin-Crini, N.; Crini, G. Desulfurization: Critical step towards enhanced selenium removal from industrial effluents. Chemosphere 2017, 117, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Wei, E.T.; Malagoli, C.; Bergomi, M.; Vivoli, G. Adverse health effects of selenium in humans. Rev. Environ. Health 2001, 16, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.M.; Adams, W.J.; Brooks, M.; Delos, C.G.; Luoma, S.N.; Maher, W.A.; Ohlendorf, H.M.; Presser, T.S.; Shaw, P. Ecological Assessment of Selenium in the Aquatic Environments; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Lemly, A.D. Symptoms and implications of selenium toxicity in fish: The Belews Lake case example. Aquat. Toxicol. 2002, 57, 39–49. [Google Scholar] [CrossRef]
- Presser, T.S. The Kesterson effect. Environ. Manag. 1994, 18, 437–454. [Google Scholar] [CrossRef]
- Barwick, M.; Maher, W. Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia. Mar. Environ. Res. 2003, 56, 471–502. [Google Scholar] [CrossRef]
- Akiho, H.; Ito, S.; Matsuda, H. Effect of oxidizing agents on selenate formation in a wet FGD. Fuel 2010, 89, 2490–2495. [Google Scholar] [CrossRef]
- Fernandez-Martinez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Biotechnol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci. Total Environ. 2015, 521C–522C, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Staicu, L.C.; van Hullebusch, E.D.; Lens, P.N.L. Industrial Selenium Pollution. Wastewater and Physical-Chemical Treatment Technologies. In Bioremediation of Selenium Contaminated Wastewaters; van Hullebusch, E.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 103–130. [Google Scholar]
- Cordoba, P.; Staicu, L.C. Flue Gas Desulfurization effluents: An unexploited selenium resource. Fuel 2018, 223, 268–276. [Google Scholar] [CrossRef]
- Kisser, J.; Wirth, M.; De Gusseme, B.; Van Eekert, M.; Zeeman, G.; Schoenborn, A.; Vinnerås, B.; Finger, D.C.; Kolbl Repinc, S.; Bulc, T.G.; et al. A review of nature-based solutions for resource recovery in cities. Blue Green Syst. 2020. [CrossRef] [Green Version]
- Critical Raw Materials. Available online: http://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en (accessed on 10 October 2019).
- Critical Raw Materials Alliance. Available online: http://criticalrawmaterials.org/barytes/ (accessed on 10 October 2019).
- Mustafa, S.; Dilara, B.; Nargis, K.; Naeem, A.; Shahida, P. Surface properties of the mixed oxides of iron and silica. Colloids Surf. A Physicochem. Eng. Asp. 2002, 205, 273–282. [Google Scholar] [CrossRef]
- Halim, C.E.; Amal, R.; Beydoun, D.; Scott, J.A.; Low, G. Evaluating the applicability of a modified toxicity characteristic leaching procedure (TCLP) for the classification of cementitious wastes containing lead and cadmium. J. Hazard. Mater. 2003, 192, 125–140. [Google Scholar] [CrossRef]
- Higgins, T.E.; Sandy, A.T.; Givens, S.W. Flue Gas Desulfurization Wastewater Treatment Primer. Power 2009. Available online: https://www.powermag.com/flue-gas-desulfurization-wastewater-treatment-primer/ (accessed on 5 October 2019).
- Staicu, L.C.; Barton, L.L. Microbial Metabolism of Selenium—For Survival or Profit. In Bioremediation of Selenium Contaminated Wastewaters; van Hullebusch, E.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–31. ISBN 978-3-319-57830-9. [Google Scholar]
- Ramaswamy, V.; Vimalathithan, R.M.; Ponnusamy, V. Synthesis and characterization of BaSO4 nano particles using micro emulsion technique. Adv. Appl. Sci. Res. 2010, 1, 197–204. [Google Scholar]
- CRC. Handbook of Chemistry and Physics, 99th ed.; Rumble, J.R., Ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Nishimura, T.; Hashimoto, H.; Nakayama, M. Removal of selenium (VI) from aqueous solution with polyamine-type weakly basic ion exchange resin. Sep. Sci. Technol. 2007, 42, 3155–3167. [Google Scholar] [CrossRef]
- Becker, U.; Fernandez-Gonzalez, A.; Prieto, M.; Harrison, R.; Putnis, A. Direct calculation of thermodynamic properties of the barite-celestite solid solution from molecular principles. Phys. Chem. Miner. 2000, 27, 291–300. [Google Scholar] [CrossRef]
- Hanor, J.S. Barite-celestine geochemistry and environments of formation. Rev. Mineral. Geochem. 2000, 40, 193–275. [Google Scholar] [CrossRef]
- Mindat. Available online: https://www.mindat.org/element/Barium (accessed on 1 September 2019).
- Tokunaga, K.; Takahashi, Y. Effective removal of selenite and selenate ions from aqueous solution by barite. Environ. Sci. Technol. 2017, 51, 9194–9201. [Google Scholar] [CrossRef]
- Manam, J.; Das, S. Thermally stimulated luminescence studies of undoped, Cu and Mn doped BaSO4 compounds. Indian J. Pure Appl. Phys. 2009, 47, 435–438. [Google Scholar]
- Prameena, B.; Anbalagan, G.; Sangeeth, V.; Gunasekaran, S.; Ramkumaar, G.R. Behaviour of Indian natural barite mineral. Int. J. ChemTech Res. 2013, 5, 220–231. [Google Scholar]
- British Geological Survey (BGS). Mineral Profiles: Barites. 2005. Available online: https://www.bgs.ac.uk/downloads/start.cfm?id=1406 (accessed on 5 October 2019).
- Han, K.N. Fundamentals of Aqueous Metallurgy. Society for Mining. In Metallurgy and Exploration; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2002; p. 197. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Effluent Limitations Guidelines and Standards for the Steam Electric Power Generating Point Source Category. Available online: https://www.epa.gov/eg/steam-electric-power-generating-effluent-guidelines-2015-final-rule (accessed on 3 November 2015).
- Ostergaard, L.F. Procedures for the Determination of Stable Elements in Construction Materials from the Nuclear Reactors at Risø National Laboratory. Riso-R-1548. Available online: https://core.ac.uk/download/pdf/13787186.pdf (accessed on 5 October 2019).
- Stack, A.G.; Raiteri, P.; Gale, J.D. Accurate rates of the complex mechanisms for growth and dissolution of minerals using a combination of rare-event theories. J. Am. Chem. Soc. 2011, 134, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.; Astilleros, J.M.; Fernández-Díaz, L. Environmental remediation by crystallization of solid solutions. Elements 2013, 9, 195–201. [Google Scholar] [CrossRef]
- American Petroleum Institute (API). Specification 13A, 18th Edition. Specification for Drilling Fluids—Specifications and Testing. Available online: https://www.api.org/certification-programs/api-monogram-program-and-apiqr/~/media/Files/Certification/Monogram-APIQR/program-updates/13A-18th-Edition-Purch-Guidlines-R1-2011-12-06.pdf (accessed on 20 August 2019).
- Sigma Aldrich. Available online: https://www.sigmaaldrich.com/catalog/product/mm/101717?lang=pl®ion=PL (accessed on 10 October 2019).
- Sigma Aldrich. Available online: https://www.sigmaaldrich.com/catalog/product/sigald/243353?lang=pl®ion=PL (accessed on 10 October 2019).
- Distripark. Available online: https://distripark.com/chlorek-baru-dwuwodny (accessed on 10 October 2019).
- Rafako. Available online: https://www.rafako.com.pl/products/640/648/products/640/648/656/674 (accessed on 20 September 2019).






| Parameter | Raw FGD | Desulfurized FGD | Change (%) |
|---|---|---|---|
| pH | 7.37 (±0.5) | 6.88 (±0.5) | −7 |
| Conductivity (mS·cm−1) | 9.89 (±0.2) | 14.86 (±0.3) | +50 |
| TOC (mg·L−1) | 51.7 (±2.9) | 38.8 (±2.2) | −25 |
| Major anions (mg·L−1) | |||
| Br−1 | 16.6 (±0.6) | 15.4 (±0.6) | −7 |
| Cl−1 | 823 (±1.9) | 5950 (±14.2) | +623 |
| F−1 | 34.6 (±1.1) | 19.5 (±0.6) | −44 |
| CO32− | 209.0 (±2.5) | 26.0 (±0.3) | −88 |
| NO3−1 | 130.0 (±30) | 77.5 (±18.6) | −40 |
| SO42− | 7090.0 (±920) | 52.6 (±6.8) | −99 |
| Major cations (mg·L−1) | |||
| Ca | 503.1 (±5.0) | 454 (±3.2) | −10 |
| K | 34.0 (±0.1) | 10.6 (±0.1) | −69 |
| Mg | 1828.1 (±33.4) | 1778.7 (±8.5) | −3 |
| Na | 258.7 (±12.3) | 240.7 (±10.4) | −7 |
| Mn | 107.9 (±1.28) | 105.3 (±0.32) | −2 |
| Element (µg·L−1) | Raw FGD | Desulfurized FGD | Change (%) |
|---|---|---|---|
| Al | 341.0 (±4.1) | 46.7 (±1.4) | −86.3 |
| B | 2009.0 (±1.0) | 2004.0 (±1.0) | −0.3 |
| Ba | 64.0 (±5.2) | 3326.0 (±53) | +5097 |
| Cd | 103.4 (±1.0) | 111.5 (±2.2) | +7.8 |
| Co | 221.2 (±3.6) | 224.3 (±1.3) | +1.4 |
| Cu | 41.7 (±0.14) | 20.0 (±0.32) | −51.9 |
| Fe | 720.6 (±43.2) | 923.0 (±24.9) | +28.0 |
| Li | 246.5 (±4.8) | 231.2 (±3.5) | −6.2 |
| Mo | 23.5 (±0.08) | 8.9 (±0.16) | −62.2 |
| Ni | 448.0 (±2.1) | 445.4 (±1.5) | −0.6 |
| Se | 615.0 (±3.8) | 374.0 (±6.3) | −39.2 |
| Si | 378.0 (±3.0) | 486.0 (±6.0) | +28.5 |
| Sr | 1446.0 (±3) | 131.0 (±5) | −90.9 |
| U | 31.2 (±0.75) | 7.8 (±0.09) | −74.9 |
| Zn | 478.0 (±3.0) | 503.0 (±2.0) | +5.23 |
| Characteristic | Recovered | Commercial |
|---|---|---|
| Size (nm) | 100–400 | >400 |
| Color | Off-white | White |
| Density (g·cm−3) | 4.5 (±0.1) | 4.5 (±0.1) |
| PZC | 6.8 (±0.1) | 6.0 (±0.1) |
| Mineralogy | Crystalline | Crystalline |
| Assay (%) | >99 | >99 |
| Recovered BaSO4 | Commercial BaSO4 | |
|---|---|---|
| Element | Weight (%) | Weight (%) |
| BaO | 64.58 (±0.43) | 64.99 (±0.37) |
| SO3 | 33.64 (±0.53) | 33.82 (±0.28) |
| SiO2 | 0.01 (±0.01) | 0.03 (±0.03) |
| Al2O3 | <0.01 | 0.01 (±0.01) |
| CaO | 0.78 (±0.07) | 0.17 (±0.02) |
| MgO | 0.14 (±0.14) | <0.01 |
| FeO | 0.01 (±0.01) | 0.01 (±0.01) |
| MnO | 0.01 (±0.01) | 0.01 (±0.01) |
| SrO | 0.02 (±0.01) | 0.26 (±0.04) |
| Na2O | 0.20 (±0.02) | 0.33 (±0.03) |
| Cl | 0.41 (±0.13) | 0.14 (±0.02) |
| Total | 99.78 | 99.78 |
| Recovered | Commercial | |||||
|---|---|---|---|---|---|---|
| Element | Associated * with Barite (µg·L−1) | Leached (µg·L−1) | % Released | Associated with Barite (µg·L−1) | Leached (µg·L−1) | % Released |
| Al | 284.3 | 29.3 (±1.43) | 10.3 | n/d | 146.7 (±10.3) | - |
| Ba | 294,200 | 617.3 (±30.1) | 0.21 | n/d | 204.2 (±7.3) | - |
| Mg | 49,400 | 52,448 (±788) | n/d | 10.7 (±2) | - | |
| Mo | 14.63 | 15.8 (±0.11) | n/d | 10.4 (±0.5) | - | |
| Na | 18,000 | 15,703 (±220) | 87.2 | n/d | 4076 (±161) | - |
| Ni | 2.8 | 24.3 (±1) | n/d | n/d | - | |
| Se | 241 | 79.4 (±3.25) | 32.9 | n/d | n/d | - |
| Sr | 1315 | 24.3 (±0.27) | 1.85 | n/d | 1150 (±34.9) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staicu, L.C.; Bajda, T.; Drewniak, L.; Charlet, L. Power Generation: Feedstock for High-Value Sulfate Minerals. Minerals 2020, 10, 188. https://doi.org/10.3390/min10020188
Staicu LC, Bajda T, Drewniak L, Charlet L. Power Generation: Feedstock for High-Value Sulfate Minerals. Minerals. 2020; 10(2):188. https://doi.org/10.3390/min10020188
Chicago/Turabian StyleStaicu, Lucian C., Tomasz Bajda, Lukasz Drewniak, and Laurent Charlet. 2020. "Power Generation: Feedstock for High-Value Sulfate Minerals" Minerals 10, no. 2: 188. https://doi.org/10.3390/min10020188
APA StyleStaicu, L. C., Bajda, T., Drewniak, L., & Charlet, L. (2020). Power Generation: Feedstock for High-Value Sulfate Minerals. Minerals, 10(2), 188. https://doi.org/10.3390/min10020188