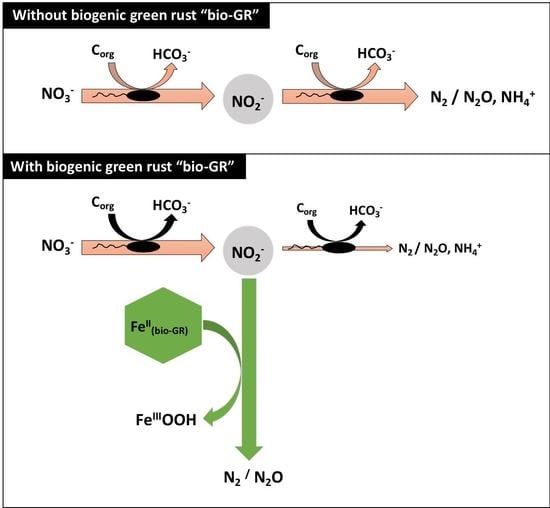
Biogenic Fe(II-III) Hydroxycarbonate Green Rust Enhances Nitrate Removal and Decreases Ammonium Selectivity during Heterotrophic Denitrification
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of Biogenic Iron(II-III) Hydroxycarbonate Green Rust Samples
3.2. Nitrate Reduction in Axenic Cultures of Shewanella spp. with and without Biogenic Iron(II-III) Hydroxycarbonate Green Rust
3.3. Nitrite Reduction by Three Strains of Shewanella Species
3.4. Nitrate and Nitrite Reduction by Autochthonous Wastewater-Denitrifying Bacteria Influent
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Philips, S.; Laanbroek, H.J.; Verstraete, W. Origin, causes and effects of increased nitrite concentrations in aquatic environments. Rev. Environ. Sci. Biotechnol. 2002, 1, 115–141. [Google Scholar] [CrossRef]
- Lorenz, M.C. A marriage of old and new: Chemostats and microarrays identify a new model system for ammonium toxicity. PLoS Biol. 2006, 4, 1905–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinn, C.; Marco, A.; Serrano, L. Influence of low levels of water salinity on toxicity of nitrite to anuran larvae. Chemosphere 2013, 92, 1154–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban, R.; Arizb, I.; Cruzb, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloern, J.E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 2001, 210, 223–253. [Google Scholar] [CrossRef]
- Teichberg, M.; Fox, S.E.; Olsen, Y.S.; Valiela, I.; Martinetto, P.; Iribarne, O.; Yuriko Muto, E.; Petti, M.A.V.; Corbisier, T.N.; Soto-Jiménez, M.; et al. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: Nutrient enrichment experiments with Ulva spp. Glob. Chang. Biol. 2010, 16, 2624–2637. [Google Scholar] [CrossRef] [Green Version]
- Akunna, J.C.; Bizeau, C.; Moletta, R. Nitrate and nitrite reductions with anaerobic sludge using various carbon sources: Glucose, glycerol, acetic acid, lactic acid and methanol. Water Res. 1993, 27, 1303–1312. [Google Scholar] [CrossRef]
- Foglar, L.; Briski, F.; Sipos, L.; Vuković, M. High nitrate removal from synthetic wastewater with the mixed bacterial culture. Bioresour. Technol. 2005, 96, 879–888. [Google Scholar] [CrossRef]
- Glass, C.; Silverstein, J. Denitrification kinetics of high nitrate concentration water: pH effect on inhibition and nitrite accumulation. Water Res. 1998, 32, 831–839. [Google Scholar] [CrossRef]
- Khan, A.; Ayub, M.; Khan, W.M. Hyperammonemia is associated with increasing severity of both liver cirrhosis and hepatic encephalopathy. Int. J. Hepatol. 2016, 2016, doi. [Google Scholar] [CrossRef] [Green Version]
- De Lucas, A.; Rodríguez, L.; Villaseñor, J.; Fernández, F.J. Denitrification potential of industrial wastewaters. Water Res. 2005, 39, 3715–3726. [Google Scholar] [CrossRef]
- Rajagopal, R.; Saady, N.M.C.; Torrijos, M.; Thanikal, J.V.; Hung, Y.-T. Sustainable agro-food industrial wastewater treatment using high rate anaerobic process. Water 2013, 5, 292–311. [Google Scholar] [CrossRef] [Green Version]
- Gander, M.; Jefferson, B.; Judd, S. Aerobic MBRs for domestic wastewater treatment: A review with cost considerations. Sep. Purif. Technol. 2000, 18, 119–130. [Google Scholar] [CrossRef]
- Zakkour, P.D.; Gaterell, M.R.; Griffin, P.; Gochin, R.J.; Lester, J.N. Anaerobic treatment of domestic wastewater in temperate climates: Treatment plant modelling with economic considerations. Water Res. 2001, 35, 4137–4149. [Google Scholar] [CrossRef]
- Aiyuk, S.; Odonkor, P.; Theko, N.; van Haandel, A.; Verstraete, W. Technical problems ensuing from UASB reactor application in domestic wastewater treatment without pre-treatment. Int. J. Environ. Sci. Dev. 2010, 1, 392–398. [Google Scholar] [CrossRef]
- Kassab, G.; Halalsheh, M.; Klapwijk, A.; Fayyad, M.; van Lier, J.B. Sequential anaerobic–aerobic treatment for domestic wastewater—A review. Bioresour. Technol. 2010, 101, 3299–3310. [Google Scholar] [CrossRef]
- Sani, A.; Scholz, M.; Bouillon, L. Seasonal assessment of experimental vertical-flow constructed wetlands treating domestic wastewater. Bioresour. Technol. 2013, 147, 585–596. [Google Scholar] [CrossRef]
- Bestawy, E.E.; AL-Hejin, A.; Amer, R.; Kashmeri, R.A. Decontamination of domestic wastewater using suspended individual and mixed bacteria in batch system. J. Bioremediat. Biodegrad. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Subtil, E.L.; Mierzwa, J.C.; Hespanhol, I. Comparison between a conventional membrane bioreactor (c-MBR) and a biofilm membrane bioreactor (bf-MBR) for domestic wastewater treatment. Braz. J. Chem. Eng. 2014, 31, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Nasr, F.A.; Mikhaeil, B. Treatment of domestic wastewater using modified septic tank. Desalin. Water Treat. 2014, 56, 2073–2081. [Google Scholar] [CrossRef]
- Nasr, F.A.; Doma, H.S.; Abdel-Halim, H.S.; El-Shafai, S.A. Chemical industry wastewater treatment. TESCE 2004, 30, 1183–1206. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Liu, M.; Wu, Q. Water quality improvement of a lagoon containing mixed chemical industrial wastewater by micro-electrolysis-contact oxidization. Appl. Phys. Eng. 2011, 12, 390–398. [Google Scholar] [CrossRef]
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. Hydroxyl radical’s role in the remediation of wastewater. J. Photochem. Photobiol. B 2012, 116, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.S.D.; Madeira, L.M.; Boaventura, R.A.R. Decontamination of an industrial cotton dyeing wastewater by chemical and biological processes. Ind. Eng. Chem. Res. 2014, 53, 2412–2421. [Google Scholar] [CrossRef] [Green Version]
- Shrimali, M.; Singh, K.P. New methods of nitrate removal from water. Environ. Pollut. 2001, 112, 351–359. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, T.; Feng, C.; Wu, H.; Deng, Z.; Wu, C.; Lu, B. Treatment of food processing wastewater in a full-scale jet biogas internal loop anaerobic fluidized bed reactor. Biodegradation 2011, 22, 347–357. [Google Scholar] [CrossRef]
- Rupani, P.F.; Singh, R.P.; Ibrahim, M.H.; Esa, N. Review of current palm oil mill effluent (POME) treatment methods: Vermicomposting as a sustainable practice. World Appl. Sci. J. 2010, 10, 1190–1201. [Google Scholar]
- Gotmare, M.; Dhoble, R.M.; Pittule, A.P. Biomethanation of dairy waste water through UASB at mesophilic temperature range. Int. J. Adv. Eng. Sci. Technol. 2011, 8, 1–9. [Google Scholar]
- Passeggi, M.; Lopez, I.; Borzacconi, L. Integrated anaerobic treatment of dairy industrial wastewater and sludge. Water Sci. Technol. 2009, 59, 501–506. [Google Scholar] [CrossRef]
- Senturk, E.; Ince, M.; Engin, O.G. Treatment efficiency and VFA composition of a thermophilic anaerobic contact reactor treating food industry wastewater. J. Hazard. Mater. 2010, 176, 843–848. [Google Scholar] [CrossRef]
- Sun, G.; Austin, D. Completely autotrophic nitrogen-removal over nitrite in lab-scale constructed wetlands: Evidence from a mass balance study. Chemosphere 2007, 68, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- Rodríguez-Maroto, J.M.; García-Herruzo, F.; García-Rubio, A.; Gómez-Lahoz, C.; Vereda-Alonso, C. Kinetics of the chemical reduction of nitrate by zero-valent iron. Chemosphere 2009, 74, 804–809. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Z.; Zhang, Z.; Yang, Y.; Xu, X. Kinetics of nitrate reductive denitrification by nanoscale zero-valent iron. Process Saf. Environ. Prot. 2010, 88, 439–445. [Google Scholar] [CrossRef]
- Ryu, A.; Jeong, S.-W.; Jang, A.; Choi, H. Reduction of highly concentrated nitrate using nanoscale zero-valent iron: Effects of aggregation and catalyst on reactivity. Appl. Catal. B 2011, 105, 128–135. [Google Scholar] [CrossRef]
- Choe, S.; Liljestrand, H.M.; Khim, J. Nitrate reduction by zero-valent iron under different pH regimes. Appl. Geochem. 2004, 19, 335–342. [Google Scholar] [CrossRef]
- Van Nooten, T.; Springael, D.; Bastiaens, L. Positive impact of microorganisms on the performance of laboratory-scale permeable reactive iron barriers. Environ. Sci. Technol. 2008, 42, 1680–1686. [Google Scholar] [CrossRef]
- Choi, J.; Batchelor, B. Nitrate reduction by fluoride green rust modified with copper. Chemosphere 2008, 70, 1108–1116. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Koch, C.B.; Nancke-Krogh, H.; Borggaard, O.K.; Sorensen, J. Abiotic nitrate reduction to ammonium: Key role of green rust. Environ. Sci. Technol. 1996, 30, 2053–2056. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Guldberg, S.; Erbs, M.; Koch, C.B. Kinetics of nitrate reduction by green rusts—Effects of interlayer anion and Fe(II):Fe(III) ratio. Appl. Clay Sci. 2001, 18, 81–91. [Google Scholar] [CrossRef]
- Guerbois, D.; Ona-Nguema, G.; Morin, G.; Abdelmoula, M.; Laverman, A.L.; Mouchel, J.-M.; Barthelemy, K.; Maillot, F.; Brest, J. Nitrite reduction by biogenic hydroxycarbonate green rusts: Evidence for hydroxy-nitrite green rust formation as intermediate reaction product. Environ. Sci. Technol. 2014, 48, 4505–4514. [Google Scholar] [CrossRef]
- Ona-Nguema, G.; Abdelmoula, M.; Jorand, F.; Benali, O.; Géhin, A.; Block, J.-C.; Génin, J.-M.R. Iron(II,III) hydroxycarbonate green rust formation and stabilisation from lepidocrocite bioreduction. Environ. Sci. Technol. 2002, 36, 16–20. [Google Scholar] [CrossRef]
- Ona-Nguema, G.; Carteret, C.; Benali, O.; Abdelmoula, M.; Génin, J.-M.R.; Jorand, F. Competitive formation of hydroxycarbonate green rust 1 versus hydroxysulphate green rust 2 in Shewanella putrefaciens cultures. Geomicrobiol. J. 2004, 21, 79–90. [Google Scholar] [CrossRef]
- Ona-Nguema, G.; Morin, G.; Wang, Y.; Menguy, N.; Juillot, F.; Olivi, L.; Aquilanti, G.; Abdelmoula, M.; Ruby, C.; Bargar, J.R.; et al. Arsenite sequestration at the surface of nano-Fe(OH)2, ferrous-carbonate hydroxide, and green-rust after bioreduction of arsenic-sorbed lepidocrocite by Shewanella putrefaciens. Geochim. Cosmochim. Acta 2009, 73, 1359–1381. [Google Scholar] [CrossRef]
- Fadrus, H.; Maly, J. Suppression of iron(III) interference in the determination of iron(II) in water by the 1,10-phenanthroline method. Analyst 1975, 100, 549–554. [Google Scholar] [CrossRef]
- Ruby, C.; Naille, S.; Ona-Nguema, G.; Morin, G.; Mallet, M.; Guerbois, D.; Barthélémy, K.; Etique, M.; Zegeye, A.; Zhang, Y.; et al. Use of ferrihydrite-coated pozzolana and biogenic green rust to purify wastewater containing phosphate and nitrate. Curr. Org. Chem. 2016, 6, 100–118. [Google Scholar]
- Venkateswaran, K.; Moser, D.P.; Dollhopf, M.E.; Lies, D.P.; Saffarini, D.A.; MacGregor, B.J.; Ringelberg, D.B.; White, D.C.; Nishijima, M.; Sano, H.; et al. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 1999, 49, 705–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, B.; Nealson, K.H. Physiology and enzymology involved in denitrification by Shewanella putrefaciens. Appl. Environ. Microbiol. 1997, 63, 2613–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-García, C.; Murray, A.E.; Klappenbach, J.A.; Stewart, V.; Tiedje, J.M. Respiratory nitrate ammonification by Shewanella oneidensis MR-1. J. Bacteriol. 2007, 189, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Samuelsson, M.-O. Dissimilatory nitrate reduction to nitrite, nitrous oxide, and ammonium by Pseudomonas putrefaciens. Appl. Environ. Microbiol. 1985, 50, 812–815. [Google Scholar] [CrossRef] [Green Version]
- Akunna, J.C.; Bizeau, C.; Moletta, R. Denitrification in anaerobic digesters: Possibilities and influence of wastewater COD/N-NOX ratio. Environ. Technol. 1992, 13, 825–836. [Google Scholar] [CrossRef]
- Cooper, D.C.; Picardal, F.W.; Schimmelmann, A.; Coby, A.J. Chemical and biological interactions during nitrate and goethite reduction by Shewanella putrefaciens 200. Appl. Environ. Microbiol. 2003, 69, 3517–3525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaszczyk, M. Effect of medium composition on the denitrification of nitrate by Paracoccus denitrificans. Appl. Environ. Microbiol. 1993, 59, 3951–3953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelso, B.; Smith, R.V.; Laughlin, R.J.; Lennox, S.D. Dissimilatory nitrate reduction in anaerobic sediments leading to river nitrite accumulation. Appl. Environ. Microbiol. 1997, 63, 4679–4685. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Peng, Y.; Wang, S.; Lu, C.; Cao, X.; Zhu, Y. Nitrite accumulation under constant temperature in anoxic denitrification process: The effects of carbon sources and COD/NO(3)-N. Bioresour. Technol. 2012, 114, 137–143. [Google Scholar] [CrossRef]
- Betlach, M.R.; Tiedje, J.M. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl. Environ. Microbiol. 1981, 42, 1074–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarova, V.; Capdeville, B.; Nikolov, L. Influence of seeding conditions on nitrite accumulation in a denitrifying fluidized bed reactor. Water Res. 1994, 28, 1189–1197. [Google Scholar] [CrossRef]
- Ruiz, G.; Jeison, D.; Rubilar, O.; Ciudad, G.; Chamy, R. Nitrification-denitrification via nitrite accumulation for nitrogen removal from wastewaters. Bioresour. Technol. 2006, 97, 330–335. [Google Scholar] [CrossRef]
- Fan, J.; Wang, W.; Zhang, B.; Guo, Y.; Ngo, H.H.; Guo, W.; Zhang, J.; Wu, H. Nitrogen removal in intermittently aerated vertical flow constructed wetlands: Impact of influent COD/N ratios. Bioresour. Technol. 2013, 143, 461–466. [Google Scholar] [CrossRef]
- Third, K.A.; Olav Sliekers, A.; Kuenen, J.G.; Jetten, M.S.M. The CANON System (Completely Autotrophic Nitrogen-removal Over Nitrite) under ammonium limitation interaction and competition. Syst. Appl. Microbiol. 2001, 24, 588–596. [Google Scholar] [CrossRef]
- Chang, X.; Li, D.; Liang, Y.; Yang, Z.; Cui, S.; Liu, T.; Zeng, H.; Zhang, J. Performance of a completely autotrophic nitrogen removal over nitrite process for treating wastewater with different substrates at ambient temperature. J. Environ. Sci. 2013, 25, 688–697. [Google Scholar] [CrossRef]
- Mosquera-Corral, A.; González, F.; Campos, J.L.; Méndez, R. Partial nitrification in a SHARON reactor in the presence of salts and organic carbon compounds. Process Biochem. 2005, 40, 3109–3118. [Google Scholar] [CrossRef]
- Hu, Z.; Lotti, T.; van Loosdrecht, M.; Kartal, B. Nitrogen removal with the anaerobic ammonium oxidation process. Biotechnol. Lett. 2013, 35, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Van Gerven, E.; Zheng, P.; Kuenen, J.G.; Jetten, M.S.M. Ammonium removal from concentrated waste streams with the anaerobic ammonium oxidation (Anammox) process in different reactor configurations. Water Res. 1997, 31, 1955–1962. [Google Scholar] [CrossRef] [Green Version]









| Origin of Carbon Compounds | COD (mg O2/L) | BOD (mg O2/L) | COD/BOD Ratio | Average ± sd | References |
|---|---|---|---|---|---|
| Agro-food industry | 4018 | 2045 | 2.0 | 1.9 ± 0.4 | [26] |
| Agro-food industry | 50,000 | 25,000 | 2.0 | [27] | |
| Agro-food industry | 1950 | 900 | 2.2 | [28] | |
| Agro-food industry | 7800 | 3450 | 2.3 | [29] | |
| Agro-food industry | 5750 | 5000 | 1.2 | [30] | |
| Agro-food industry | 10,496 | 6300 | 1.7 | [31] | |
| Domestic wastewater | 925 | 440 | 2.1 | 2.4 ± 0.8 | [18] |
| Domestic wastewater | 912 | 289 | 3.2 | [19] | |
| Domestic wastewater | 285.2 | 71.4 | 4.0 | [17] | |
| Domestic wastewater | 960 | 450 | 2.1 | [20] | |
| Domestic wastewater | 1420 | 900 | 1.6 | [16] | |
| Domestic wastewater | 300 | 160 | 1.9 | [13] | |
| Domestic wastewater | 522 | 208 | 2.5 | [15] | |
| Domestic wastewater | 550 | 250 | 2.2 | [14] | |
| Chemical industry | 340.7 | 8.9 | 38.5 | 30 ± 23 | [22] |
| Chemical industry | 2912 | 150 | 19.4 | [21] | |
| Chemical industry | 495 | 127.5 | 3.9 | [24] | |
| Chemical industry | 4566 | 80 | 57.1 | [23] |
| Experiments | Mass of Bio-GR (g) | [NO3−] (mg-N L−1) | [NO2−] (mg-N L−1) | [NH4+] (mg-N L−1) | Initial pH | Bacterial Density (CFU mL−1) |
|---|---|---|---|---|---|---|
| 1 bio-GR + nitrate | 0.1 | 75(2) | 0 | 0 | 6.5 | 0 |
| 1 bio-GR + nitrate | 0.1 | 76(0) | 0 | 0 | 7.5 | 0 |
| 1 bio-GR + nitrate | 0.1 | 76(0) | 0 | 0 | 10.0 | 0 |
| 2 Strain ATCC 12099 + nitrate | – | 89(4) | 0 | 0 | 7.5 | 7 × 109 |
| 1 bio-GR + Strain ATCC 12099 + nitrate | 0.1 | 83(0) | 0 | 0 | 7.5 | 7 × 109 |
| 1 Strain ATCC 12099 + nitrite | – | 0 | 58(14) | 0 | 7.5 | 7 × 109 |
| 2 Strain ATCC 8071 + nitrate | – | 83(3) | 0 | 0 | 7.5 | 7 × 109 |
| 1 bio-GR + Strain ATCC 8071 + nitrate | 0.1 | 83(0) | 0 | 0 | 7.5 | 7 × 109 |
| 1 Strain ATCC 8071 + nitrite | – | 0 | 66(13) | 0 | 7.5 | 7 × 109 |
| 1 Strain MR-1 + nitrate | – | 92(4) | 0 | 0 | 7.5 | 7 × 109 |
| 1 bio-GR + Strain MR-1 + nitrate | 0.1 | 83(0) | 0 | 0 | 7.5 | 7 × 109 |
| 2 Strain MR-1 + nitrite | – | 0 | 72(2) | 0 | 7.5 | 7 × 109 |
| 3 Wastewater influents + nitrate | – | 73 | 2.2 | 55 | 7.5 | n.d |
| 3 bio-GR + nitrate + Wastewater influents | 0.1 | 84 | 0.1 | 52 | 7.5 | n.d |
| 3 Wastewater influents + nitrite | – | 0 | 74 | 61 | 7.5 | n.d |
| 2 bio-GR + nitrite + Wastewater influents | 0.1 | 0 | 77(0) | 71(18) | 7.5 | n.d |
| Experiments Performed with Nitrate in the Starting Solution | ||||||
|---|---|---|---|---|---|---|
| X(NO3−)t (%) | R(NH4+)t (%) | R(NO2−)t (%) | S(NH4+)t (%) | S(NO2−)t (%) | Gas (%) | |
| 1 Strain ATCC 12099 | 100(0) | 37(5) | 12(1) | 37(5) | 12(1) | 51(6) |
| 2 bio-GR(CO3) + Strain ATCC 12099 | 100(0) | 28(3) | 2(3) | 28(3) | 2(3) | 70(5) |
| 1 Strain ATCC 8071 | 100(0) | 31(3) | 28(9) | 31(3) | 28(9) | 41(11) |
| 2 bio-GR(CO3) + Strain ATCC 8071 | 100(0) | 11(1) | 13(5) | 11(1) | 13(5) | 76(4) |
| 2 Strain MR-1 | 100(0) | 42(12) | 0(0) | 42(12) | 0(0) | 58(12) |
| 2 bio-GR(CO3) + Strain MR-1 | 99(1) | 4(6) | 7(2) | 4(6) | 7(2) | 88(7) |
| 3 Wastewater influent | 40 | 4 | 1 | 10 | 2 | 28 |
| 3 bio-GR(CO3) + Wastewater influent | 100 | 1 | 6 | 1 | 6 | 93 |
| Experiments Performed with Nitrite in the Starting Solution | ||||||
| X(NO2−)t (%) | R(NH4+)t (%) | S(NH4+)t (%) | Gas (%) | |||
| 2 Strain ATCC 12099 | 84(1) | 51(9) | 60(12) | 24(13) | ||
| 2 Strain ATCC 8071 | 74(1) | 51(6) | 69(6) | 5(5) | ||
| 1 Strain MR-1 | 100(0) | 78(16) | 78(16) | 22(16) | ||
| 3 Wastewater influent | 42 | −25 | −60 | n.d | ||
| 1 bio-GR(CO3) + Wastewater influent | 96(3) | 4(3) | 5(3) | 87(9) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ona-Nguema, G.; Guerbois, D.; Pallud, C.; Brest, J.; Abdelmoula, M.; Morin, G. Biogenic Fe(II-III) Hydroxycarbonate Green Rust Enhances Nitrate Removal and Decreases Ammonium Selectivity during Heterotrophic Denitrification. Minerals 2020, 10, 818. https://doi.org/10.3390/min10090818
Ona-Nguema G, Guerbois D, Pallud C, Brest J, Abdelmoula M, Morin G. Biogenic Fe(II-III) Hydroxycarbonate Green Rust Enhances Nitrate Removal and Decreases Ammonium Selectivity during Heterotrophic Denitrification. Minerals. 2020; 10(9):818. https://doi.org/10.3390/min10090818
Chicago/Turabian StyleOna-Nguema, Georges, Delphine Guerbois, Céline Pallud, Jessica Brest, Mustapha Abdelmoula, and Guillaume Morin. 2020. "Biogenic Fe(II-III) Hydroxycarbonate Green Rust Enhances Nitrate Removal and Decreases Ammonium Selectivity during Heterotrophic Denitrification" Minerals 10, no. 9: 818. https://doi.org/10.3390/min10090818
APA StyleOna-Nguema, G., Guerbois, D., Pallud, C., Brest, J., Abdelmoula, M., & Morin, G. (2020). Biogenic Fe(II-III) Hydroxycarbonate Green Rust Enhances Nitrate Removal and Decreases Ammonium Selectivity during Heterotrophic Denitrification. Minerals, 10(9), 818. https://doi.org/10.3390/min10090818