Interaction between Microbes, Minerals, and Fluids in Deep-Sea Hydrothermal Systems
Abstract
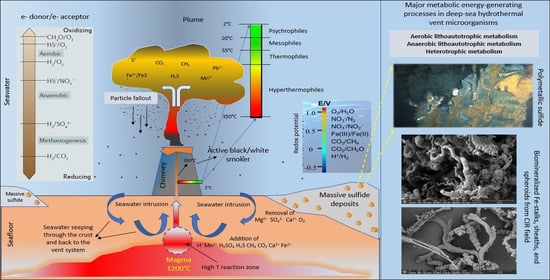
:1. Introduction
2. Microbial Diversity in Deep-Sea Hydrothermal Vent Systems
2.1. Sulfur Oxidizers and Sulfate Reducers
2.2. Methanogens and Methane Oxidizers
2.3. Hydrogen-Oxidizers
2.4. Ammonium-Oxidizers and Nitrate-Reducers
2.5. Iron-Oxidizers and Iron-Reducers
3. Biomineralization and Microbe–Mineral Interactions
3.1. Iron-Metabolizers and Biomineralization
3.2. Role of Microbes in the Precipitation of Mn-Oxides and Si in SWIR Hydrothermal Deposits
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lonsdale, P. Clustering of suspension feeding macrobenthos near abyssal hydrothermal vents at oceanic spreading centers. Deep Sea Res. A 1977, 24, 857–858. [Google Scholar] [CrossRef]
- Jannasch, H.W.; Wirsen, C.O. Chemosynthetic primary production at East Pacific sea floor spreading centers. Bioscience 1979, 29, 592–598. [Google Scholar] [CrossRef]
- Beaulieu, S.E.; Baker, E.T.; German, C.R. Where are the undiscovered hydrothermal vents on oceanic spreading ridges? Deep Sea Res. Part II Top Stud. Oceanogr. 2015, 121, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.T.; German, C.R. Mid-Ocean Ridges: Hydrothermal Interactions between the Litho-Sphere And Oceans; German, C., Lin, J., Parson, L.M., Eds.; American Geophysical Union: Washington, DC, USA, 2004; pp. 245–266. [Google Scholar]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.J.; Bach, W.; McCollom, T.M. Geomicrobiology in oceanography: Microbe–mineral interactions at and below the seafloor. Trends Microbiol. 2005, 13, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Amend, J.P.; Mccollom, T.M.; Hentscher, M.; Bach, W. Catabolic and anabolic energy for chemo-lithoautotrophs in deep-sea hydrothermal systems hosted in different rock types. Geochim. Cosmochim. Acta 2011, 75, 5736–5748. [Google Scholar] [CrossRef]
- Jannasch, H.W.; Mottl, M.J. Geomicrobiology of deep-sea hydrothermal vents. Science 1985, 229, 717–725. [Google Scholar] [CrossRef]
- Frank, K.L.; Rogers, D.R.; Olins, H.C.; Vidoudez, C.; Girguis, P.R. Characterizing the distribution and rates of microbial sulfate reduction at Middle Valley hydrothermal vents. ISME J. 2013, 7, 1391–1401. [Google Scholar] [CrossRef]
- McDermott, J.M.; Sylva, S.P.; Ono, S.; German, C.R.; Seewald, J.S. Abiotic redox reactions in hydrothermal mixing zones: Decreased energy availability for the subsurface biosphere. Proc. Natl. Acad. Sci. USA 2020, 117, 20453–20461. [Google Scholar] [CrossRef]
- Kelley, D.S. Methane-bearing fluids in the oceanic crust: Gabbro-hosted fluid inclusions from the southwest Indian ridge. J. Geophys. Res. 1996, 101, 2943–2962. [Google Scholar] [CrossRef]
- Konn, C.; Charlou, J.L.; Holm, N.G.; Mousis, O. The production of methane, hydrogen, and organic compounds in ultramafic-hosted hydrothermal vents of the Mid-Atlantic Ridge. Astrobiology 2015, 15, 381–399. [Google Scholar] [CrossRef]
- Sievert, S.M.; Vetriani, C. Chemoautotrophy at deep-sea vents: Past, present, and future. Oceanography 2012, 25, 218–233. [Google Scholar] [CrossRef]
- Anderson, R.E.; Sogin, M.L.; Baross, J.A. Biogeography and ecology of the rare and abundant mi-crobial lineages in deep-sea hydrothermal vents. FEMS Microbiol. Ecol. 2015, 91, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, J.; Zhang, Y.; Jiang, H.; Leng, H.; Xiao, X. Microbial community structure of deep-sea hydro-thermal vents on the ultraslow spreading Southwest Indian Ridge. Front. Microbiol. 2017, 8, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Gonnella, G.; Adam, N.; Schippers, A.; Burkhardt, L.; Kurtz, S.; Schwarz-Schampera, U.; Franke, H.; Perner, M. Hydrothermal chimneys host habitat-specific microbial communities: Analogues for studying the possible impact of mining seafloor massive sulfide deposits. Sci. Rep. 2018, 8, 10386. [Google Scholar] [CrossRef]
- Dick, G.J. The microbiomes of deep-sea hydrothermal vents: Distributed globally, shaped locally. Nat. Rev. Microbiol. 2019, 17, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Chu, F.; Yu, X.; Li, X.; Tao, C. Lipid biomarkers reveal microbial communities in hydrothermal chimney structures from the 49.6ºE hydrothermal vent field at the Southwest Indian Ocean Ridge. Geomicrobiol. J. 2016, 34, 557–566. [Google Scholar] [CrossRef]
- Hu, J.F.; Meyers, P.A.; Chen, G.K.; Peng, P.A.; Yang, Q.H. Archaeal and bacterial glycerol dialkyl glycerol tetraethers in sediments from the Eastern Lau Spreading Center, South Pacific Ocean. Org. Geochem. 2012, 43, 162–167. [Google Scholar] [CrossRef]
- Jaeschke, A.; Eickmann, B.; Lang, S.Q.; Bernasconi, S.M.; Strauss, H.; Früh-Green, G.L. Biosignatures in chimney structures and sediment from the Loki’s Castle low-temperature hydrothermal vent field at the Arctic Mid-Ocean Ridge. Extremophiles 2014, 18, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.E.; Campbell, J.H.; Kirshtein, J.D.; Meneghin, J.; Podar, M.; Steinberg, J.I.; Seewald, J.S.; Tivey, M.K.; Voytek, M.A.; Yang, Z.K.; et al. Microbial community structure of hydrothermal deposits from geochemically different vent fields along the Mid-Atlantic Ridge. Environ. Microbiol. 2011, 13, 2158–2171. [Google Scholar] [CrossRef]
- McCollom, T.M. Geochemical constraints on primary productivity in submarine hydrothermal vent plumes. Deep-Sea Res. I 2000, 47, 85–101. [Google Scholar] [CrossRef]
- Shock, E.L.; Holland, M.E. Geochemical energy sources that support the subsurface biosphere. In The Subsurface Biosphere at Mid-Ocean Ridges; Wilcock, R.W., et al., Eds.; American Geophysical Union: Washington, DC, USA, 2004; pp. 153–166. [Google Scholar]
- Peng, X.T.; Chen, S.; Zhou, H.Y.; Zhang, L.X.; Wu, Z.J.; Li, J.T.; Li, J.W.; Xu, H.C. Diversity of biogenic minerals in low-temperature Si-rich deposits from a newly discovered hydrothermal field on the ultraslow spreading Southwest Indian Ridge. J. Geophys. Res. 2011, 116, G03030. [Google Scholar] [CrossRef] [Green Version]
- Takai, K.; Gamo, T.; Tsunogai, U.; Nakayama, N.; Hirayama, H.; Nealson, K.H.; Horikoshi, K. Geochemical and microbiological evidence for a hydrogen-based, hyperthermophilic subsurface lithoautotrophic microbial ecosystem (HyperSLiME) beneath an active deep-sea hydrothermal field. Extremophile 2004, 8, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Karson, J.A.; Früh-Green, G.L.; Yoerger, D.R.; Shank, T.M.; Butterfield, D.A.; Hayes, J.M.; Schrenk, M.O.; Olson, E.J.; Proskurowski, G. A Serpentinite-Hosted Ecosystem: The Lost City Hydrothermal Field. Science 2005, 307, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, M.; Lindblom, S.; Broman, C.; Holm, H.G. Fossilized microorganisms associated with zeolite–carbonate interfaces in sub-seafloor hydrothermal environments. Geobiology 2008, 6, 155–170. [Google Scholar] [CrossRef] [PubMed]
- McCollom, T.M. Geochemical constraints on source of metabolic energy for chemolithoautotrophy in ultramafic-hosted deep-sea hydrothermal systems. Astrobiology 2007, 7, 933–950. [Google Scholar] [CrossRef]
- Takai, K.; Nunoura, T.; Horikoshi, K.; Shibuya, T.; Nakamura, K.; Suzuki, Y.; Stott, M.; Massoth, G.J.; Christenson, B.W.; de Ronde, C.E.J.; et al. Variability in microbial communities in black smoker chimneys at the NW caldera vent field, Brothers volcano, Kermadec arc. Geomicrobiol. J. 2009, 26, 552–569. [Google Scholar] [CrossRef]
- Zhou, H.; Li, J.; Peng, X.; Meng, J.; Wang, F.; Ai, Y. Microbial diversity of a sulfide black smoker in main endeavour hydrothermal vent field, Juan de Fuca Ridge. J. Microbiol. 2009, 47, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Dahle, H.; Roalkvam, I.; Thorseth, I.H.; Pedersen, R.B.; Steen, I.H. The versatile in situ gene expression of an Epsilonproteobacteria-dominated biofilm from a hydrothermal chimney. Environ. Microbiol. Rep. 2013, 5, 282–290. [Google Scholar] [CrossRef]
- Perner, M.; Gonnella, G.; Kurtz, S.; LaRoche, J. Handling temperature bursts reaching 464 °C: Different microbial strategies in the Sisters Peak hydrothermal chimney. Appl. Environ. Microbiol. 2014, 80, 4585–4598. [Google Scholar] [CrossRef] [Green Version]
- Takai, K.; Kobayashi, H.; Nealson, K.H.; Horikoshi, K. Deferribacter desulfuricans sp. nov., a novel sulfur-, nitrate-and arsenate-reducing thermophile isolated from a deep-sea hydrothermal vent. Int. J. Syst. Evol. 2003, 53, 839–846. [Google Scholar] [CrossRef]
- Takai, K.; Nakagawa, S.; Sako, Y.; Horikoshi, K. Balnearium lithotrophicum gen. nov., sp. nov., a novel thermophilic, strictly anaerobic, hydrogen-oxidizing chemolithoautotroph isolated from a black smoker chimney in the Suiyo Seamount hydrothermal system. Int. J. Syst. Evol. 2003, 53, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Inagaki, F.; Nakagawa, S.; Hirayama, H.; Nunoura, T.; Sako, Y.; Nealson, K.H.; Horikoshi, K. Isolation and phylogenetic diversity of members of previously uncultivated ε-Proteobacteria in deep-sea hydrothermal fields. FEMS Microbiol. Lett. 2003, 218, 167–174. [Google Scholar]
- Inagaki, F.; Takai, K.; Kobayashi, H.; Nealson, K.H.; Horikoshi, K. Sulfurimonas autotrophica gen. nov., sp. nov., a novel sulfuroxidizing ε-proteobacterium isolated from hydrothermal sediments in the Mid-Okinawa Trough. Int. J. Syst. Evol. Microbiol. 2003, 53, 1801–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, F.; Takai, K.; Nealson, K.H.; Horikoshi, K. Sulfurovum lithotrophicum gen. nov., sp. nov., a novel sulfur-oxidizing chemolithoautotroph within the ε-Proteobacteria isolated from Okinawa Trough hydrothermal sediments. Int. J. Syst. Evol. Microbiol. 2004, 54, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Hirayamam, H.; Nakagawa, T.; Suzuki, Y.; Nealson, K.H.; Horikoshi, K. Lebetimonas acidiphila gen. nov., sp. nov., a novel thermophilic, acidophilic, hydrogen-oxidizing chemolithoautotroph within the “Epsilonproteobacteria”, isolated from a deep-sea hydrothermal fumarole in the Mariana Arc. Int. J. Syst. Evol. Microbiol. 2005, 55, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Akerman, N.H.; Butterfield, D.A.; Huber, J.A. Phylogenetic diversity and functional gene patterns of sulfur-oxidizing subseafloor Epsilonproteobacteria in diffuse hydrothermal vent fluids. Front. Microbiol. 2013, 4, 185. [Google Scholar] [CrossRef] [Green Version]
- Sylvan, J.B.; Toner, B.M.; Edwards, K.J. Life and death of deep-sea vents: Bacterial diversity and ecosystem succession on inactive hydrothermal sulfides. MBio 2012, 3, e00279-11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.T.; Zhou, H.Y.; Li, J.T.; Li, J.W.; Chen, S.; Yao, H.Q.; Wu, Z.J. Intracellular and extracellular mineralization of a microbial community in the Edmond deep-sea vent field environment. Sediment. Geol. 2010, 229, 193–206. [Google Scholar] [CrossRef]
- Nunoura, T.; Oida, H.; Miyazaki, M.; Suzuki, Y.; Takai, K.; Horikoshi, K. Desulfothermus okinawensis sp. nov., a thermophilic and heterotrophic sulfate-reducing bacterium isolated from a deep-sea hydrothermal field. Int. J. Syst. Evol. Microbiol. 2007, 57, 2360–2364. [Google Scholar] [CrossRef]
- Cao, J.; Birien, T.; Gayet, N.; Huang, Z.; Shao, Z.; Jebbar, M.; Alain, K. Desulfurobacterium indicum sp. nov., a thermophilic sulfur reducing bacterium from the Indian Ocean. Int. J. Syst. Evol. Microbiol. 2017, 67, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sievert, S.; Wang, Y.; Seewald, J.S.; Wang, F.; Xiao, X. Microbial succession during the transition from active to inactive stages of deep-sea hydrothermal vent sulfide chimneys. Microbiome 2020, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Nunoura, T.; Miyazaki, M.; Suzuki, Y.; Takai, K.; Horikoshi, K. Hydrogenivirga okinawensis sp. nov., a thermophilic sulfur-oxidizing chemolithoautotroph isolated from a deep-sea hydrothermal field, Southern Okinawa Trough. Int. J. Syst. Evol. Microbiol. 2008, 58, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Cha, I.; Roh, S.W.; Kim, S.; Hong, H.; Lee, H.; Lim, W.; Rhee, S. Desulfotomaculum tongense sp. nov., a moderately thermophilic sulfate-reducing bacterium isolated from a hydrothermal vent sediment collected from the Tofua Arc in the Tonga Trench. Antonie Van Leeuwenhoek 2013, 104, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Pjevac, P.; Bach, W.; Markert, S.; Schweder, T.; Jamieson, J.; Petersen, S.; Amann, R.; Meyerdierks, A. Microbial metal sulfide oxidation in inactive hydrothermal vent chimneys suggested by metagenomic and metaproteomic analyses. Environ. Microbiol. 2019, 21, 682–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birrien, J.; Zeng, X.; Jebbar, M.; Cambonbonavita, M.; Querellou, J.; Oger, P.; Bienvenu, N.; Xiao, X.; Pri-eur, D. Pyrococcus yayanosii sp. nov., an obligate piezophilic hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2011, 61, 2827–2881. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Birrien, J.; Fouquet, Y.; Cherkashov, G.; Jebbar, M.; Querellou, J.; Oger, P.; Cambon-Bonavita, M.A.; Xiao, X.; Prieur, D. Pyrococcus CH1, an obligate piezophilic hyperthermophile: Extending the upper pressure–temperature limits for life. ISME J. 2009, 3, 873–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callac, N.; Oger, P.; Lesongeur, F.; Rattray, J.E.; Vannier, P.; Michoud, G.; Beauverger, M.; Gayet, N.; Rouxel, O.; Jebbar, M.; et al. Pyrococcus kukulkanii sp. nov., a hyperthermophilic, piezophilic archaeon isolated from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2016, 66, 3142–3149. [Google Scholar] [CrossRef] [Green Version]
- Blochl, E.; Rachel, R.; Burggraf, S.; Hafenbradl, D.; Jannasch, H.W.; Stetter, K.O. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113 °C. Extremophiles 1997, 1, 14–21. [Google Scholar]
- Takai, K.; Suzuki, M.; Nakagawa, S.; Miyazaki, M.; Suzuki, Y.; Inagaki, F.; Horikoshi, K. Sulfurimonas paralvinellae sp. nov., a novel mesophilic, hydrogen- and sulfur-oxidizing chemolithoautotroph within the Epsilonproteobacteria isolated from a deep-sea hydrothermal vent polychaete nest, reclassification of Thiomicrospira denitrificans as Sulfurimonas denitrificans comb. nov. and emended description of the genus Sulfurimonas. Int. J. Syst. Evol. Microbiol. 2006, 56, 1725–1733. [Google Scholar]
- Brazelton, W.; Nelson, B.; Schrenk, M. Metagenomic evidence for H2 oxidation and H2 production by serpentinite-hosted subsurface microbial communities. Front. Microbiol. 2012, 2, 268. [Google Scholar] [CrossRef] [Green Version]
- Jeanthon, C.; Lharidon, S.; Reysenbach, A.; Corre, E.; Vernet, M.; Messner, P.; Sleytr, U.B.; Prieur, D. Methanococcus vulcanius sp. nov., a novel hyperthermophilic methanogen isolated from East Pacif-ic Rise, and identification of Methanococcus sp. DSM 4213T as Methanococcus fervens sp. nov. Int. J. Syst. Evol. Microbiol. 1999, 49, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Jeanthon, C.; L’Haridon, S.; Reysenbach, A.L.; Vernet, M.; Messner, P.; Sleytr, U.B.; Prieur, D. Methanococcus infernus sp. nov., a novel hyperthermophilic lithotrophic methanogen isolated from a deep-sea hydrothermal vent. Int. J. Syst. Bacteriol. 1998, 48, 913–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellack, A.; Huber, H.; Rachel, R.; Wanner, G.; Wirth, R. Methanocaldococcus villosus sp. nov., a heavily flagellated archaeon that adheres to surfaces and forms cell–cell contacts. Int. J. Syst. Evol. Microbiol. 2011, 61, 1239–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, L.C.; Jung, J.; Kim, Y.; Kwon, S.; Park, C.; Holden, J.F. Methanocaldococcus bathoardescens sp. nov., a hyperthermophilic methanogen isolated from a volcanically active deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2015, 65, 1280–1283. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.J.; Leigh, J.A.; Mayer, F.; Woese, C.R.; Wolfe, R.S. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 1983, 136, 254–261. [Google Scholar] [CrossRef]
- Kurr, M.; Huber, R.; Konig, H.; Jannasch, H.W.; Fricke, H.; Trincone, A.; Kristjansson, J.K.; Stetter, K.O. Methanopyrus kandleri, gen. and sp. nov. represents a novel group of hyperthermophilic methanogens, growing at 110 C. Arch. Microbiol. 1991, 156, 239–247. [Google Scholar] [CrossRef]
- Takai, K.; Inoue, A.; Horikoshi, K. Methanothermococcus okinawensis sp. nov., a thermophilic, methane-producing archaeon isolated from a Western Pacific deep-sea hydrothermal vent system. Int. J. Syst. Evol. Microbiol. 2002, 52, 1089–1095. [Google Scholar]
- Wankel, S.D.; Adams, M.M.; Johnston, D.T.; Hansel, C.M.; Joye, S.B.; Girguis, P.R. Anaerobic methane oxidation in metalliferous hydrothermal sediments: Influence on carbon flux and decoupling from sulfate reduction. Environ. Microbiol. 2012, 14, 2726–2740. [Google Scholar] [CrossRef]
- Nakagawa, S.; Takai, K. Deep-sea vent chemoautotrophs: Diversity, biochemistry and ecological significance. FEMS Microbiol. Ecol. 2008, 65, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Adam, N.; Perner, M. Microbially mediated hydrogen cycling in deep-sea hydrothermal vents. Front. Microbiol. 2018, 9, 2873. [Google Scholar] [CrossRef]
- Miroshnichenko, M.L.; Lharidonm, S.; Schumann, P.; Spring, S.; Bonch-Osmolovskaya, E.A.; Jeanthon, C.; Stackebrandt, E. Caminibacter profundus sp. nov., a novel thermophile of Nautiliales ord. nov. within the class ‘Epsilonproteobacteria’, isolated from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2004, 54, 41–45. [Google Scholar] [CrossRef]
- Xu, W.; Li, M.; Ding, J.; Gu, J.; Luo, Z. Bacteria dominate the ammonia-oxidizing community in a hydrothermal vent site at the mid-atlantic ridge of the South Atlantic Ocean. Appl. Microbiol. Biotechnol. 2014, 98, 7993–8004. [Google Scholar] [CrossRef]
- Christakis, C.A.; Polymenakou, P.N.; Mandalakis, M.; Nomikou, P.; Kristoffersen, J.B.; Lampridou, D.; Kotoulas, G.; Magoulas, A. Microbial community differentiation between active and inactive sulfide chimneys of the Kolumbo submarine volcano, Hellenic Volcanic Arc. Extremophiles 2018, 22, 13–27. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Peng, X.; Wu, Z.; Chen, S.; Fang, J. Microbial diversity and biomineralization in low-temperature hydrothermal iron-silica-rich precipitates of the Lau Basin hydrothermal field. FEMS Microbiol. Ecol. 2012, 81, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Pitcher, A.; Rychlik, N.; Hopmans, E.C.; Spieck, E.; Rijpstra, W.I.C.; Ossebaar, J.; Schouten, S.; Wagner, M.; Sinninghe Damsté, J.S. Crenarchaeol dominates the membrane lipids of Candidatus Nitrososphaera gargensis, a thermophilic group I.1b Archaeon. ISME J. 2010, 4, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, A.; Wuchter, C.; Siedenberg, K.; Schouten, S.; Sinninghe Damsté, J.S. Crenarchaeol tracks winter blooms of ammonia-oxidizing Thaumarchaeota in the coastal North Sea. Limnol. Oceanogr. 2011, 56, 2308–2318. [Google Scholar] [CrossRef] [Green Version]
- Alain, K.; Rolland, S.; Crassous, P.; Lesongeur, F.; Zbinden, M.; Le Gall, C.; Godfroy, A.; Page, A.; Juniper, S.K.; Cambonbonavita, M.; et al. Desulfurobacterium crinifex sp.nov., a novel thermophilic, pinkish-streamer forming, chemolithoautotrophic bacterium isolated from a Juan de Fuca Ridge hydrothermal vent and amendment of the genus Desulfurobacterium. Extremophiles 2003, 7, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Yamaguchi, K.; Hanada, S. Sulfurovum denitrificans sp. nov., an obligately chemolithoautotrophic sulfur-oxidizing epsilonproteobacterium isolated from a hydrothermal field. Int. J. Syst. Evol. Microbiol. 2018, 68, 2183–2187. [Google Scholar] [CrossRef]
- Cao, J.; Gayet, N.; Zeng, X.; Shao, Z.; Jebbar, M.; Alain, K. Pseudodesulfovibrio indicus gen. nov., sp. nov., a piezophilic sulfate reducing bacterium from the Indian Ocean and reclassification of four spe-cies of the genus Desulfovibrio. Int. J. Syst. Evol. Microbiol. 2016, 66, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; McAllister, S.; Leavitt, A.; Emerson, D.; Moyer, C.L.; Glazer, B.T. Fe-oxidizing microbes are hydrothermal vent ecosystem engineers at the Loihi Seamount. In AGU Fall Meeting Abstracts; Abstract B14B-01; American Geophysical Union: Washington, DC, USA, 2013. [Google Scholar]
- Emerson, D.; Moyer, C.L. Neutrophilic Fe-oxidizing bacteria are abundant at the Loihi Seamount hydrothermal vents and play a major role in Fe oxide deposition. Appl. Environ. Microbiol. 2002, 68, 3085–3093. [Google Scholar] [CrossRef] [Green Version]
- Emerson, D.; Rentz, J.A.; Liburn, T.G.; Davis, R.E.; Aldrich, H.; Chan, C.; Moyer, C.L. A novel line-age of Proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS ONE 2007, 2, e667. [Google Scholar] [CrossRef] [Green Version]
- Makita, H.; Tanaka, E.; Mitsunobu, S.; Miyazaki, M.; Nunoura, T.; Uematsu, K.; Takaki, Y.; Nishi, S.; Shimamura, S.; Takai, K. Mariprofundus micogutta sp. nov., a novel ironoxidizing zetaproteobacterium isolated from a deep-sea hydrothermal field at the Bayonnaise knoll of the Izu-Ogasawara arc, and a description of Mariprofundales ord. nov. and Zetaproteobacteria classis nov. Arch. Microbiol. 2017, 199, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.J.; Rogers, D.R.; Wirsen, C.O.; McCollom, T.M. Isolation and characterization of novel psychrophilic, neutrophilic, Fe-oxidizing, chemolithoautotrophic α- and γ-Proteobacteria from the deep sea. Appl. Environ. Microbiol. 2003, 69, 2906–2913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Peng, X.; Zhou, H.; Li, J.; Sun, Z. Molecular evidence for microorganisms participating in Fe, Mn, and S biogeochemical cycling in two low-temperature hydrothermal fields at the Southwest Indian Ridge. J. Geophys. Res. Biogeosci. 2013, 118, 665–679. [Google Scholar] [CrossRef]
- Toner, B.M.; Marcus, M.A.; Edwards, K.J.; Rouxel, O.; German, C.R. Measuring the form of iron in hydrothermal plume particles. Oceanography 2012, 25, 209–212. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Fang, J.; Xie, W.; Peng, X.; Dong, L.; Wu, Z.; Zhou, H. Aerobic and anaerobic ammonia-oxidizing microorganisms in low-temperature hydrothermal Fe-Si-rich precipitates of the south-western Pacific Ocean. Geomicrobiol. J. 2013, 31, 42–52. [Google Scholar] [CrossRef]
- Slobodkina, G.; Reysenbach, A.L.; Panteleeva, A.; Kostrikina, N.; Wagner, I.; Bonch-Osmolovskaya, E.; Slobodkin, A.I. Deferrisoma camini gen. nov., sp. nov., a moderately thermophilic, dissimilatory iron (III)-reducing bacterium from a deep-sea hydrothermal vent that forms a distinct phylogenetic branch in the Deltaproteobacteria. Int. J. Syst. Evol. Microbiol. 2012, 62, 2463–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashefi, K.; Lovley, D.R. Extending the upper temperature limit for life. Science 2003, 301, 934. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.G.; Fyfe, W.W.; Beveridge, T.J. Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chem. Geol. 1987, 63, 225–232. [Google Scholar] [CrossRef]
- Emerson, D.; Moyer, C.L. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl. Environ. Microbiol. 1997, 63, 4784–4792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerson, D.; Weiss, J.V. Bacterial iron oxidation in circumneutral freshwater habitats: Findings from the field and the laboratory. Geomicrobiol. J. 2004, 21, 405–414. [Google Scholar] [CrossRef]
- Chan, C.S.; Fakra, S.C.; Emerson, D.; Fleming, E.J.; Edwards, K.J. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: Implication for biosignature formation. ISME J. 2011, 5, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Su, L.; Wang, F.; Yang, J.; Gu, L.; Sun, M.; Li, Q.; Zhou, H.; Fang, J. Elucidating the biomineralization of low-temperature hydrothermal precipitates with varying Fe, Si contents: Indication from ultrastructure and microbiological analyses. Deep-Sea Res. I 2020, 157, 103208. [Google Scholar] [CrossRef]
- Emerson, D.; Fleming, E.J.; McBeth, J.M. Iron-oxidizing bacteria: An environmental and genomic perspective. Annu. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E.J.; Cetinic, I.; Chan, C.S.; King, D.W.; Emerson, D. Ecological succession among iron-oxidizing bacteria. ISME J. 2014, 8, 804–815. [Google Scholar] [CrossRef]
- Frankel, R.B.; Bazylinski, D.A. Biologically induced mineralization by bacteria. Rev. Mineral. Geochem. 2003, 54, 95–114. [Google Scholar] [CrossRef]
- Grimes, S.T.; Brock, F.; Rickard, D.; Davies, K.L.; Edwards, D.; Briggs, D.E.G.; Parkes, R.J. Under-standing fossilization: Experimental pyritization of plants. Geology 2001, 29, 123–126. [Google Scholar] [CrossRef]
- Vidyalakshmi, R.; Paranthaman, R.; Bhakyaraj, R. Sulphur oxidizing bacteria and pulse nutrition—A review. World J. Agric. Sci. 2009, 5, 270–278. [Google Scholar]
- Fortin, D.; Langley, S. Formation and occurrence of biogenic iron-rich minerals. Earth Sci. Rev. 2005, 72, 1–19. [Google Scholar] [CrossRef]
- Edwards, K.J.; Bazylinski, D.A. Intracellular minerals and metal deposits in prokaryotes. Geobiology 2008, 6, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R. Magnetotactic bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Dobeneck, T.; Vali, H. Fossil bacterial magnetite in deep sea sediments from the South Atlantic Ocean. Nature 1986, 320, 611–615. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Zhang, W.; Zhang, W.; Zhao, Y.; Xiao, T.; Wu, L.-F.; Pan, H. The detection of magnetotactic bacteria in deep sea sediments from the east Pacific Manganese Nodule Province. Environ. Microbiol. Rep. 2016, 8, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, M.; Suzina, N.; Kudryashova, E.; Ariskina, E. New magnet-sensitive structures in bacterial and archaeal cells. Biol. Cell 2002, 94, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Vainshtein, M.; Suzina, N.; Sorokin, V. A new type of magnet-sensitive inclusions in cells of photosynthetic bacteria. Syst. Appl. Microbiol. 1997, 20, 182–186. [Google Scholar] [CrossRef]
- Vadas, A.; Monbouquette, H.G.; Johnson, E.; Schröder, I. Identification and characterization of a novel ferric reductase from the hyperthermophilic archaeon Archaeoglobus fulgidus. J. Biol. Chem. 1999, 274, 36715–36721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, H.-J.; Johnson, E.; Schröder, I.; Rees, D.C. Crystal structures of a novel ferric reductase from the hyperthermophilic archaeon Archaeoglobus fulgidus and its complex with NADPþ. Structure 2001, 9, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Juniper, S.K.; Martineau, P.; Sarrazin, J.; Gelinas, Y. Microbial-mineral floc associated with nascent hydrothermal activity on CoAxial segment, Juan de Fuca Ridge. Geophys. Res. Lett. 1995, 22, 179–182. [Google Scholar] [CrossRef]
- Holden, J.F.; Adams, M.W.W. Microbe–metal interactions in marine hydrothermal environments. Curr. Opin. Chem. Biol. 2003, 7, 160–165. [Google Scholar] [CrossRef]
- Takai, K.; Nakamura, K. Compositional physiological and metabolic variability in microbial communities associated with geochemically diverse deep-sea hydrothermal vent fluids. In Geomicrobiology: Molecular and Environmental Perspective; Barton, L.L., Mandl, M., Loy, A., Eds.; Springer: Berlin, Germany, 2010; pp. 251–283. [Google Scholar]
- Ver Eecke, H.C.; Kelley, D.S.; Holden, J.F. Abundances of hyperthermophilic autotrophic Fe(III) oxide reducers and heterotrophs in hydrothermal sulfide chimneys of the northeastern Pacific Ocean. Appl. Environ. Microbiol. 2009, 75, 242–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandernack, K.W.; Tebo, B.M. Manganese scavenging and oxidation at hydrothermal vents and in vent plumes. Geochim. Cosmochim. Acta 1993, 57, 3907–3923. [Google Scholar] [CrossRef]
- Spilde, M.N.; Northup, D.E.; Boston, P.J.; Schelble, R.T.; Dano, K.E.; Crossey, L.J.; Dahm, C.N. Geomicrobiology of cave ferromanganese deposits: A field and laboratory investigation. Geomicrobiol. J. 2005, 22, 99–116. [Google Scholar] [CrossRef]
- Miller, A.Z.; Dionísio, A.; Braga, M.S.; Hernández-Mariné, M.; Afonso, M.J.; Muralha, V.S.F.; Herrera, L.K.; Raabe, J.; Fernandez-Cortes, A.; Cuezva, S.; et al. Biogenic Mn oxide minerals coating in a subsurface granite environment. Chem. Geol. 2012, 322–323, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Dekov, V.M.; Kamenov, G.D.; Stmmeyer, J.; Thirty, M.; Savelli, C.; Shanks, W.C.; Fortin, D.; Kuzmann, E.; Vertes, A. Hydrothermal nontronite formation at Eolo Seamount (aeolian volcanic arc, Tyrrhenian Sea). Chem. Geol. 2007, 245, 103–119. [Google Scholar] [CrossRef]
- Ueshima, M.; Tazaki, K. Possible role of microbial polysaccharides nontronite formation. Clays Clay Miner. 2001, 49, 292–299. [Google Scholar] [CrossRef]
- Köhler, B.; Singer, A.; Stoffers, O. Biogenic nontronite from marine white smoker chimneys. Clays Clay Miner. 1994, 42, 689–701. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Jones, B.; Phoenix, V.R.; Ferris, F.G.; Renaut, R.W. The microbial role in hot spring silicification. AMBIO 2004, 33, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; de Ronde, C.E.J.; Renaut, R.W. Mineralized microbes from Giggenbach submarine volcano. J. Geophys. Res. 2008, 113, B08S05. [Google Scholar] [CrossRef] [Green Version]
- Halbach, M.; Halbach, P.; Lüders, V. Sulfide-impregnated and pure silica precipitates of hydrothermal origin from the Central Indian Ocean. Chem. Geol. 2002, 182, 357–375. [Google Scholar] [CrossRef]
- Jones, B.; Konhauser, K.O.; Renaut, R.W.; Wheeler, R.S. Microbial silicification in Iodine Pool, Waimangu geothermal area, North Island, New Zealand: Implications for recognition and identification of ancient silicified microbes. J. Geol. Soc. 2004, 161, 983–993. [Google Scholar] [CrossRef]
- Kompanichenko, V.N. Changeable hydrothermal media as potential cradle of life on a planet. Planet. Space Sci. 2009, 57, 468–476. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Turner, D. In vitro growth of iron sulfide chimneys: Possible culture chambers for origin-of-life experiments. Terra Nova 1989, 1, 238–241. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Martin, W. Serpentinization as a source of energy for the origin of life. Geobiology 2010, 8, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Al-Hanbali, H.S.; Sowerby, S.J.; Holm, N.G. Biogenicity of silicified microbes from a hydrothermal system: Relevance to the search for evidence of life on earth and other planets. Earth Planet. Sci. Lett. 2001, 191, 213–218. [Google Scholar] [CrossRef]
- Hofmann, B.A.; Farmer, J.D.; Von Blanckenburg, F.; Fallick, A.E. Subsurface Filamentous Fabrics: An evaluation of origins based on morphological and geochemical criteria, with implications for exopaleontology. Astrobiology 2008, 8, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Rouxel, O.; Toner, B.; Germain, Y.; Glazer, B. Geochemical and iron isotopic insights into hydrothermal iron oxyhydroxide deposit formation at Loihi Seamount. Geochim. Cosmochim. Acta 2018, 220, 449–482. [Google Scholar] [CrossRef] [Green Version]
- Dauphus, N.; John, S.G.; Rouxel, O. Iron isotope systematics. Rev. Mineral. Geochem. 2017, 82, 415–510. [Google Scholar] [CrossRef]



| Categories (Bacteria and Archaea) | Main Energy-Yielding Process | Field Locations |
|---|---|---|
| Campylobacteria | S-oxidation, S-reduction, H-oxidation, and nitrate-reduction | |
| Sulfurimonas | S-oxidation, H-oxidation, and nitrate-reduction | JFR; Loki–s Castle, MAR; Suiyo Seamount; SWIR; EPR; and Kairei, Pelagia, CIR |
| Sulfurovum | ||
| Lebetimonas | ||
| Caminibacter | S-reduction, nitrate-reduction, and H-oxidation | EPR; SWIR; MAR; and JFR |
| Nautilia | ||
| Lebetimonas | ||
| Cetia | ||
| Gammaproteobacteria | S-oxidation, N-reduction, and H-oxidation | |
| Thiomicrospira | S-oxidation, N-reduction, and H-oxidation | CIR and MAR |
| Methylococcaceae | S-oxidation and N-reduction | |
| Thiotrichaceae | S-oxidation and N-reduction | SWIR |
| Ectothiorhodospiraceae | ||
| Thiohalophilus | ||
| Piscirickettsiaceae | ||
| Deltaproteobacteria | S-reduction, H-oxidation, and Fe-reduction, | |
| Desulfovibrio | S-reduction | EPR; SWIR; and SOT |
| Desulfonauticus | ||
| Desulfothermus | ||
| Desulfonauticus | H-oxidation | Rainbow, MAR |
| Deferrisoma | Fe-reduction | Eastern Lau Spreading Center |
| Zetaproteobacteria | Fe-oxidation | |
| Mariprofundus | Fe-oxidation | Loihi Seamount and Lau Basin |
| Betaproteobacteria | Ammonium-oxidation | |
| Nitrospira | Ammonium-oxidation | SWIR |
| Nitrococcus | ||
| Nitrosomonas | ||
| Aquificae | S-reduction, H-oxidation, and N-reduction | |
| Desulfobacterium | S-reduction | SWIR |
| H-oxidation | Eastern Lau Spreading Center | |
| N-reduction | JFR | |
| Firmicutes | S-reduction | |
| Desulfohalotomaculum | S-reduction | Tonga Trench |
| Euryarchaeota | Methanogenesis, methane-oxidation, and S-reduction | |
| Methanococcales | Methanogenesis | Kairei, Pelagia, CIR; Rainbow, MAR; and LCHF |
| Methanocaldococcus | ||
| Methanosarcinales | ||
| Methanopyrus | ||
| Pyrococcus | S-reduction | |
| ANME-1 | Methane-oxidation | LCHF |
| Crenarchaeota | S-reduction and Fe-reduction | |
| Pyrolobus | S-reduction | |
| Archaeoglobacae | Fe-reduction | JFR |
| Pyrodictiacceae | ||
| Thaumoarchaeota | Ammonium-oxidation | SWIR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgupta, S.; Peng, X.; Ta, K. Interaction between Microbes, Minerals, and Fluids in Deep-Sea Hydrothermal Systems. Minerals 2021, 11, 1324. https://doi.org/10.3390/min11121324
Dasgupta S, Peng X, Ta K. Interaction between Microbes, Minerals, and Fluids in Deep-Sea Hydrothermal Systems. Minerals. 2021; 11(12):1324. https://doi.org/10.3390/min11121324
Chicago/Turabian StyleDasgupta, Shamik, Xiaotong Peng, and Kaiwen Ta. 2021. "Interaction between Microbes, Minerals, and Fluids in Deep-Sea Hydrothermal Systems" Minerals 11, no. 12: 1324. https://doi.org/10.3390/min11121324
APA StyleDasgupta, S., Peng, X., & Ta, K. (2021). Interaction between Microbes, Minerals, and Fluids in Deep-Sea Hydrothermal Systems. Minerals, 11(12), 1324. https://doi.org/10.3390/min11121324