Structure Refinement and Thermal Stability Studies of the Uranyl Carbonate Mineral Andersonite, Na2Ca[(UO2)(CO3)3]·(5+x)H2O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Occurence
2.2. Chemical Composition
2.3. Single-Crystal X-ray Diffraction Study
2.4. High-Temperature Powder X-ray Diffraction Study
3. Results
3.1. Structure Description of Andersonite
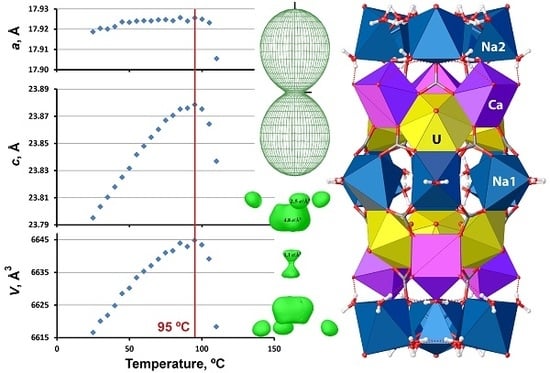
3.2. High-Temperature Behavior of Andersonite
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alwan, A.K.; Williams, P.A. The aqueous chemistry of uranium minerals. Part 2. Minerals of the liebigite group. Mineral. Mag. 1980, 43, 665–667. [Google Scholar] [CrossRef]
- Clark, D.L.; Hobart, D.E.; Neu, M.P. Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry. Chem. Rev. 1995, 95, 25–48. [Google Scholar] [CrossRef]
- Plášil, J. Oxidation–hydration weathering of uraninite: The current state-of-knowledge. J. Geosci. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Baker, R.J. Uranium minerals and their relevance to long term storage of nuclear fuels. Coord. Chem. Rev. 2014, 266–267, 123–136. [Google Scholar] [CrossRef]
- Driscoll, R.J.P.; Wolverson, D.; Mitchels, J.M.; Skelton, J.M.; Parker, S.C. A Raman spectroscopic study of uranyl minerals from Cornwall, UK. RSC Adv. 2014, 4, 59137–59149. [Google Scholar] [CrossRef] [Green Version]
- Plášil, J.; Čejka, J.; Sejkora, J.; Hloušek, J.; Škoda, R.; Novák, M.; Dušek, M.; Císařová, I.; Němec, I.; Ederová, J. Línekite, K2Ca3[(UO2)(CO3)3]2·8H2O, a new uranyl carbonate mineral from Jachymov, Czech Republic. J. Geosci. 2017, 62, 201–213. [Google Scholar] [CrossRef]
- Teterin, Y.A.; Baev, A.S.; Bogatov, S.A. X-ray photoelectron study of samples containing reactor fuel from “lava” and products growing on it which formed at Chernobyl NPP due to the accident. J. Electron. Spectrosc. Relat. Phenom. 1994, 68, 685–694. [Google Scholar] [CrossRef]
- Burakov, B.E.; Strykanova, E.E.; Anderson, E. Secondary uranium minerals on the surface of Chernobyl “Lava”. Mat. Res. Soc. Symp. Proc. 1996, 465, 1309–1311. [Google Scholar] [CrossRef]
- Olds, T.A.; Plášil, J.; Kampf, A.R.; Simonetti, A.; Sadergaski, L.R.; Chen, Y.-S.; Burns, P.C. Ewingite: Earth’s most complex mineral. Geology 2017, 45, 1007–1010. [Google Scholar] [CrossRef]
- Olds, T.A.; Plášil, J.; Kampf, A.R.; Dal Bo, F.; Burns, P.C. Paddlewheelite, a new uranyl carbonate from the Jáchymov district, Bohemia, Czech Republic. Minerals 2018, 8, 511. [Google Scholar] [CrossRef]
- Finch, R.J.; Cooper, M.A.; Hawthorne, F.C.; Ewing, R.C. Refinement of the crystal structure of rutherfordine. Can. Mineral. 1999, 37, 929–938. [Google Scholar]
- Mereiter, K. The crystal structure of liebigite, Ca2UO2(CO3)3·~11H2O. Tscher. Mineral. Petrog. Mitt. 1982, 30, 277–288. [Google Scholar] [CrossRef]
- Axelrod, J.M.; Grimaldi, F.S.; Milton, C.; Murata, K.J. The uranium minerals from the Hillside mine, Yavapai County, Arizona. Am. Mineral. 1951, 36, 1–22. [Google Scholar]
- Stefaniak, E.A.; Alsecz, A.; Frost, R.; Mathe, Z.; Sajo, I.E.; Torok, S.; Worobiec, A.; Van Grieken, R. Combined SEM/EDX and micro-Raman spectroscopy analysis of uranium minerals from a former uranium mine. J. Hazard Mater. 2009, 168, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coda, A.; Della Giusta, A.; Tazzoli, V. The structure of synthetic andersonite, Na2Ca[UO2(CO3)3].xH2O (x = 5.6). Acta Cryst. 1981, B37, 1496–1500. [Google Scholar] [CrossRef]
- Mereiter, K. Neue kristallographische Daten ueber das Uranmineral Andersonit. Anz. Österr Akad. Wiss. Math.-Naturwiss. K 1986, 123, 39–41. [Google Scholar]
- Vochten, R.; van Haverbeke, L.; van Springel, K.; Blaton, N.; Peeters, M. The structure and physicochemical characteristics of a synthetic phase compositionally intermediate between liebigite and andersonite. Can. Mineral. 1994, 32, 553–561. [Google Scholar]
- Plášil, J.; Čejka, J. A note on the molecular water content in uranyl carbonate mineral andersonite. J. Geosci. 2015, 60, 181–187. [Google Scholar] [CrossRef]
- Coda, A. Ricerche sulla struttura cristallina dell’Andersonite. Atti Accad. Naz. Lincei Rend. Cl. Sci. Fis. Mat. Nat. Ser. 1963, 34, 299–304. [Google Scholar]
- Čejka, J.; Urbanec, Z.; Čejka, J., Jr. To the crystal chemistry of andersonite. Neu. Jb. Mineral. Mh. 1987, 11, 488–501. [Google Scholar]
- De Neufville, J.P.; Kasdan, A.; Chimenti, R.J.L. Selective detection of uranium by laser-induced fluorescence: A potential remote-sensing technique. 1: Optical characteristics of uranyl geologic targets. Appl. Opt. 1981, 20, 1279–1296. [Google Scholar] [CrossRef] [PubMed]
- Amayri, S.; Arnold, T.; Reich, T.; Foerstendorf, H.; Geipel, G.; Bernhard, G.; Massanek, A. Spectroscopic characterization of the uranium carbonate andersonite Na2Ca[UO2(CO3)3]·6H2O. Environ. Sci. Technol. 2004, 38, 6032–6036. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Carmody, O.; Ertickson, K.L.; Weier, M.L.; Čejka, J. Molecular structure of the uranyl mineral andersonite—A Raman spectroscopic study. J. Mol. Struct. 2004, 703, 47–54. [Google Scholar] [CrossRef]
- Čejka, J. To the chemistry of andersonite and thermal composition of dioxo-tricarbonatouranates. Coll. Czech. Chem. Commun. 1969, 34, 1635–1656. [Google Scholar] [CrossRef]
- Čejka, J.; Urbanec, Z. Thermal and infrared spectrum analyses of natural and synthetic andersonites. J. Therm. Anal. 1988, 33, 389–394. [Google Scholar] [CrossRef]
- Vochten, R.; Van Haverbeke, L.; Van Springel, K. Synthesis of liebigite and andersonite, and study of their thermal behavior and luminescence. Can. Mineral. 1993, 31, 167–171. [Google Scholar]
- Kubatko, K.-A.; Helean, K.B.; Navrotsky, A.; Burns, P.C. Thermodynamics of uranyl minerals: Enthalpies of formation of rutherfordine, UO2CO3, andersonite, Na2CaUO2(CO3)3(H2O)5, and grimselite, K3NaUO2(CO3)3H2O. Am. Mineral. 2005, 90, 1284–1290. [Google Scholar] [CrossRef]
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Cryst. 1995, A51, 887–897. [Google Scholar] [CrossRef]
- CrysAlisPro Software System, version 1.171.38.46; Rigaku Oxford Diffraction: Oxford, UK, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Burns, P.C.; Ewing, R.C.; Hawthorne, F.C. The crystal chemistry of hexavalent uranium: Polyhedron geometries, bond-valence parameters, and polymerization of polyhedra. Can. Mineral. 1997, 35, 1551–1570. [Google Scholar]
- Wood, R.M.; Palenik, G.J. Bond valence sums in coordination chemistry. Sodium−oxygen complexes. Inorg. Chem. 1999, 38, 3926–3930. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Cryst. 2015, B71, 561–578. [Google Scholar] [CrossRef]
- Bruker, A.X.S. Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS: Karlsruhe, Germany, 2009. [Google Scholar]
- Langreiter, T.; Kahlenberg, V. TEV—A Program for the Determination and Visualization of the Thermal Expansion Tensor from Diffraction Data; Institute of Mineralogy and Petrography, University of Innsbruck: Innsbruck, Austria, 2014. [Google Scholar]
- Krivovichev, S.V.; Burns, P.C. Actinide compounds containing hexavalent cations of the VI group elements (S, Se, Mo, Cr, W). In Structural Chemistry of Inorganic Actinide Compounds; Krivovichev, S.V., Burns, P.C., Tananaev, I.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 95–182. [Google Scholar]
- Lussier, A.J.; Lopez, R.A.K.; Burns, P.C. A revised and expanded structure hierarchy of natural and synthetic hexavalent uranium compounds. Can. Mineral. 2016, 54, 177–283. [Google Scholar] [CrossRef]
- Li, Y.-P.; Krivovichev, S.V.; Burns, P.C. The crystal structure of Na4(UO2)(CO3)3 and its relationship to schroekingerite. Mineral. Mag. 2001, 65, 297–304. [Google Scholar] [CrossRef]
- Zachariasen, W.H. Crystal chemical studies of the 5f-series of elements. IV. The crystal structure of CaUO4 and SrUO4. Acta Crystallogr. 1948, 1, 281–285. [Google Scholar] [CrossRef]
- Kovba, L.M.; Ippolitova, E.A.; Simanov, Y.P.; Spitsyn, V.I. The X-ray investigation of uranates of alkali elements. Dokl. Akad. Nauk SSSR 1958, 120, 1042–1044. (In Russian) [Google Scholar]
- Chippindale, A.M.; Dickens, P.G.; Harrison, W.T.A. A Structural study of the sodium (V) uranate, NaUO3, by time-of-flight powder neutron diffraction. J. Solid State Chem. 1989, 78, 256–261. [Google Scholar] [CrossRef]
- Spitsyn, V.I.; Shi-Khua, V.; Kovba, L.M. Investigation of mixed sodium and calcium uranates. Vestn. Moskovskogo Univ. Ser. 2 Khimiya 1962, 5, 60–62. (In Russian) [Google Scholar]
- Filatov, S.K. Visokotemperaturnaia Kristallohimia (High-Temperature Crystal Chemistry); Nedra: Leningrad, Russia, 1990. (In Russian) [Google Scholar]
- Hazen, R.M.; Downs, R.T. (Eds.) Reviews in Mineralogy and Geochemistry: High-Temperature and High-Pressure Crystal Chemistry; Mineralogical Society of America: Washington, DC, USA, 2001; Volume 41, p. 596. [Google Scholar]
- Filatov, S.K. General concept of increasing crystal symmetry with an increase in temperature. Crystallogr. Rep. 2011, 56, 953–961. [Google Scholar] [CrossRef]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Kuz’mina, M.A.; Leoni, M.; Frank-Kamenetskays, O.V. Hydrated calcium oxalates: Crystal structures, thermal stability and phase evolution. Cryst. Growth Des. 2018, 18, 5465–5478. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth. Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]




| Crystal System | Trigonal |
|---|---|
| Space group | R−3m |
| a (Å) | 17.8448(4) |
| c (Å) | 23.6688(6) |
| V (Å3) | 6527.3(3) |
| Formula Mass | 631.25 |
| Size (mm3) | 0.38 × 0.27 × 0.25 |
| μ (mm–1) | 11.691 |
| Z | 18 |
| 2θ range, ° | 4.34–55.00 |
| Dcalc (g/cm3) | 2.891 |
| Total number of reflections | 11,925 |
| Unique number of reflections | 1836 |
| Unique I ≥ 2σ(I) | 1697 |
| Rint | 0.0342 |
| Rσ | 0.0218 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0182, wR2 = 0.0382 |
| Final R indexes [all data] | R1 = 0.0215, wR2 = 0.0390 |
| s | 1.121 |
| ρmin, ρmin, e/Å3 | −0.958, 0.945 |
| CSD | 1877782 |
| Atom | x | y | z | Ueq | s.o.f. | BVS * |
|---|---|---|---|---|---|---|
| U | 0.55663(2) | 0.44337(2) | 0.67846(2) | 0.00420(7) | 1 | 6.18 |
| Na1 | 0.51663(6) | 0.48337(6) | 0.82964(7) | 0.0070(4) | 1 | 1.13 |
| Na2 | 0.54058(7) | 0.45942(7) | 0.44251(8) | 0.0131(4) | 1 | 0.93 |
| Ca | 0.45594(3) | 0.54406(3) | 0.94394(4) | 0.00413(18) | 1 | 2.21 |
| C1 | 0.59021(15) | 0.40979(15) | 0.56575(19) | 0.0071(9) | 1 | 3.98 |
| C2 | 0.6832(2) | 0.5986(2) | 0.73519(13) | 0.0049(6) | 1 | 3.99 |
| O1 | 0.59553(11) | 0.40447(11) | 0.51354(13) | 0.0088(4) | 1 | 1.63 |
| O2 | 0.52559(15) | 0.35307(15) | 0.59583(9) | 0.0088(4) | 1 | 2.14 |
| O3 | 0.60320(14) | 0.55322(15) | 0.75196(9) | 0.0079(5) | 1 | 1.97 |
| O4 | 0.69945(15) | 0.57427(15) | 0.68723(9) | 0.0072(5) | 1 | 2.04 |
| O5 | 0.59310(14) | 0.58736(14) | 0.90360(9) | 0.0049(4) | 1 | 1.92 |
| O6 | 0.60695(11) | 0.39305(11) | 0.71534(13) | 0.0103(7) | 1 | 1.67 |
| O7 | 0.50574(11) | 0.49426(11) | 0.64221(14) | 0.0109(7) | 1 | 1.80 |
| OW1 | 0.43997(11) | 0.56003(11) | 0.83858(14) | 0.0049(4) | 0.928(5) | 0.40 |
| HW1 | 0.459(3) | 0.6150(13) | 0.8367(17) | 0.015(11) | 0.928(5) | - |
| OW2 | 0.58955(11) | 0.41045(11) | 0.86128(14) | 0.0098(7) | 1 | 0.19 |
| HW2A | 0.598(2) | 0.402(2) | 0.8967(12) | 0.026(18) | 1 | - |
| HW2B | 0.564(4) | 0.357(3) | 0.845(3) | 0.04(3) | 0.5 | - |
| OW3 | 0.5924(2) | 0.5924(2) | 0.500000 | 0.0116(7) | 1 | 0.28 |
| HW3 | 0.611(3) | 0.6376(19) | 0.4778(14) | 0.017(11) | 1 | - |
| OW4 | 0.4728(2) | 0.3219(2) | 0.39299(12) | 0.0166(8) | 0.881(6) | 0.16 |
| HW4 | 0.4155(14) | 0.296(4) | 0.396(3) | 0.06(2) | 0.881(6) | - |
| OW4A | 0.5289(17) | 0.3323(14) | 0.3882(9) | 0.0166(8) | 0.119(6) | 0.13 |
| HW4A | 0.497(3) | 0.334(7) | 0.3590(11) | 0.05(3) | 0.5605 | - |
| HW4B | 0.488(2) | 0.2806(12) | 0.399(2) | 0.082 | 0.5605 | - |
| OW5 | 0.666667 | 0.333333 | 0.4217(6) | 0.089(8) | 0.76(3) | - |
| HW5 | 0.6946(4) | 0.3054(4) | 0.434(3) | 0.134 | 0.507(19) | - |
| OW5A | 0.666667 | 0.333333 | 0.354(4) | 0.089(8) | 0.072(5) | - |
| HW5C | 0.638000 | 0.362000 | 0.360701 | 0.134 | 0.048(4) | - |
| OW5B | 0.7265(14) | 0.2735(14) | 0.4583(17) | 0.0049(4) | 0.072(5) | - |
| HW5A | 0.737499 | 0.262500 | 0.490549 | 0.007 | 0.072(5) | - |
| HW5B | 0.697500 | 0.302500 | 0.465120 | 0.007 | 0.072(5) | - |
| Bond | This Work | Coda et al. [15] | Mereiter [16] | |
|---|---|---|---|---|
| U–O6 | 1.784(3) | 1.776(14) | 1.775(5) | |
| U–O7 | 1.792(3) | 1.808(14) | 1.780(5) | |
| <U–OUr> | 1.788 | 1.80 | 1.778 | |
| U–O2 | ×2 | 2.416(2) | 2.414(14) | 2.413(3) |
| U–O3 | ×2 | 2.435(2) | 2.440(17) | 2.433(4) |
| U–O4 | ×2 | 2.458(2) | 2.461(18) | 2.460(4) |
| <U–Oeq> | 2.436 | 2.44 | 2.435 | |
| Ca–O2 | ×2 | 2.304(2) | 2.311(17) | 2.324(5) |
| Ca–O4 | ×2 | 2.396(2) | 2.384(15) | 2.404(4) |
| Ca–O5 | ×2 | 2.368(2) | 2.364(16) | 2.370(4) |
| Ca–OW1 | 2.542(3) | 2.557(18) | 2.555(6) | |
| <Ca–O> | 2.383 | 2.38 | 2.393 | |
| Na1–O3 | ×2 | 2.323(3) | 2.300(17) | 2.336(4) |
| Na1–O5 | ×2 | 2.416(3) | 2.464(16) | 2.437(4) |
| Na1–OW1 | 2.379(4) | 2.429(13) | 2.398(5) | |
| Na1–OW2 | 2.375(4) | 2.402(13) | 2.378(5) | |
| <Na1–O> | 2.372 | 2.39 | 2.387 | |
| Na2–O1 | 2.390(4) | 2.427(19) | 2.419(6) | |
| Na2–O7 | 2.464(4) | 2.47(2) | 2.498(6) | |
| Na2–OW3 | ×2 | 2.479(3) | 2.531(16) | 2.487(5) |
| Na2–OW4 | ×2 | 2.427(3) | 2.42(3) | 2.434(7) |
| Na2–OW4A | ×2 | 2.52(2) | ||
| <Na2–O> | 2.463 | 2.47 | 2.460 | |
| C1–O1 | 1.247(5) | 1.30(6) | 1.245(7) | |
| C1–O2 | ×2 | 1.301(3) | 1.27(4) | 1.289(6) |
| C2–O3 | 1.302(4) | 1.31(3) | 1.300(6) | |
| C2–O4 | 1.299(4) | 1.30(3) | 1.295(6) | |
| C2–O5 | 1.255(4) | 1.26(3) | 1.243(7) |
| Temp., °C | α11 | α22 | α33 |
|---|---|---|---|
| 25 | 12.6 | 12.6 | 72.1 |
| 50 | 6.7 | 6.7 | 59.8 |
| 75 | 0.9 | 0.9 | 47.6 |
| 95 | −3.7 | −3.7 | 37.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Izatulina, A.R.; Sigmon, G.E.; Krivovichev, S.V.; Burns, P.C. Structure Refinement and Thermal Stability Studies of the Uranyl Carbonate Mineral Andersonite, Na2Ca[(UO2)(CO3)3]·(5+x)H2O. Minerals 2018, 8, 586. https://doi.org/10.3390/min8120586
Gurzhiy VV, Krzhizhanovskaya MG, Izatulina AR, Sigmon GE, Krivovichev SV, Burns PC. Structure Refinement and Thermal Stability Studies of the Uranyl Carbonate Mineral Andersonite, Na2Ca[(UO2)(CO3)3]·(5+x)H2O. Minerals. 2018; 8(12):586. https://doi.org/10.3390/min8120586
Chicago/Turabian StyleGurzhiy, Vladislav V., Maria G. Krzhizhanovskaya, Alina R. Izatulina, Ginger E. Sigmon, Sergey V. Krivovichev, and Peter C. Burns. 2018. "Structure Refinement and Thermal Stability Studies of the Uranyl Carbonate Mineral Andersonite, Na2Ca[(UO2)(CO3)3]·(5+x)H2O" Minerals 8, no. 12: 586. https://doi.org/10.3390/min8120586
APA StyleGurzhiy, V. V., Krzhizhanovskaya, M. G., Izatulina, A. R., Sigmon, G. E., Krivovichev, S. V., & Burns, P. C. (2018). Structure Refinement and Thermal Stability Studies of the Uranyl Carbonate Mineral Andersonite, Na2Ca[(UO2)(CO3)3]·(5+x)H2O. Minerals, 8(12), 586. https://doi.org/10.3390/min8120586