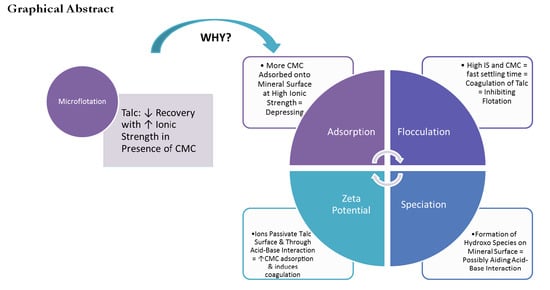
The Effect of the Ionic Strength of Process Water on the Interaction of Talc and CMC: Implications of Recirculated Water on Floatable Gangue Depression
Abstract
:1. Introduction
2. Materials & Methods
2.1. Materials
2.2. Depressant Preparation
2.3. Synthetic Plant Water Preparation
2.4. Microflotation
2.5. Adsorption Studies
2.6. Zeta Potential Tests
2.7. Flocculation Tests
3. Results
3.1. The Microflotation Response of Talc to Process Water Ionic Strength and CMC Dosages
3.2. Investigating the Adsorption of CMC onto Talc with Increased Ionic Strength of SPW
3.3. Effect of Ionic Strength and pH on the Zeta Potential of Talc Particles
3.4. Effect of Ionic Strength and pH on the Inorganic Electrolyte Speciation
3.5. Effect of Ionic Strength on the Settling Time of Talc Particles
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Connor, C.; Wiese, J.; Corin, K.; McFadzean, B. On the Management of Gangue Minerals in the Flotation of Platinum Group Minerals. Min. Metall. Explor. 2019, 36, 55–62. [Google Scholar] [CrossRef]
- Shortridge, P.G.; Harris, P.J.; Bradshaw, D.J. The influence of ions on the effectiveness of polysaccharide depressants in the flotation of talc. In Polymers in Mineral Processing, Proceedings of the 3rd UBC-McGill Bi-Annual International Symposium on Fundamentals of Mineral Processing, Quebec City, QC, Canada, 22–26 August 1999; Laskowski, J.S., Ed.; CIM: Quebec City, QC, Canada, 1999; pp. 155–169. [Google Scholar]
- Pugh, R.J. Macromolecular organic depressants in suiphide flotation—A review, 2. Theoretical analysis of the forces involved in the depressant action. Int. J. Miner. Process. 1989, 25, 131–146. [Google Scholar] [CrossRef]
- Jenkins, P.; Ralston, J. Adsorption of a polysaccharide at the talc-aqueous solution interface. Colloids Surf. A Physicochem. Eng. Asp. 1998, 139, 27–40. [Google Scholar] [CrossRef]
- Parolis, L.A.S.; van der Merwe, R.; Groenmeyer, G.V.; Harris, P.J. The influence of metal cations on the behaviour of carboxymethyl celluloses as talc depressants. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 109–115. [Google Scholar] [CrossRef]
- Morris, G.E.; Fornasiero, D.; Ralston, J. Polymer depressants at the talc-water interface: Adsorption isotherm, microflotation and electrokinetic studies. Int. J. Miner. Process. 2002, 67, 211–227. [Google Scholar] [CrossRef]
- Pawlik, M.; Laskowski, J.S.; Ansari, A. Effect of carboxymethylcellulose and ionic strength on the stability of mineral suspensions in a potash ore flotation system. J. Colloid Interface Sci. 2003, 260, 251–258. [Google Scholar] [CrossRef]
- Burdukova, E.; Van Leerdam, G.C.; Prins, F.E.; Smeink, R.G.; Bradshaw, D.J.; Laskowski, J.S. Effect of calcium ions on the adsorption of CMC onto the basal planes of New York talc—A ToF-SIMS study. Miner. Eng. 2008, 21, 1020–2025. [Google Scholar] [CrossRef]
- Feng, Q.M.; Feng, B.; Lu, Y.P. Influence of copper ions and calcium ions on adsorption of CMC on chlorite. Trans. Nonferr. Met. Soc. China (Engl. Ed.) 2013, 23, 237–242. [Google Scholar] [CrossRef]
- Becker, M.; Harris, P.J.; Wiese, J.G.; Bradshaw, D.J. Mineralogical characterisation of naturally floatable gangue in Merensky reef ore flotation. Int. J. Miner. Process. 2009, 246–255. [Google Scholar] [CrossRef]
- Allison, S.A.; O’Connor, C.T. An investigation into the flotation behaviour of pyrrhotite. Miner. Eng. 2011, 98, 202–207. [Google Scholar] [CrossRef]
- Hodgson, M.; Agar, G. Electrochemical investigations into the flotation chemistry of pentlandite and pyrrhotite: Process water and xanthate interactions. Can. Metall. Q. 1989, 28, 189–198. [Google Scholar] [CrossRef]
- Biçak, Ö.; Ekmekçi, Z.; Can, M.; Öztürk, Y. The effect of water chemistry on froth stability and surface chemistry of the flotation of a Cu-Zn sulfide ore. Int. J. Miner. Process. 2012, 102–103, 32–37. [Google Scholar] [CrossRef]
- Ikumapayi, F.; Makitalo, M.; Johansson, B.; Rao, K.H. Recycling of process water in sulphide flotation: Effect of calcium and sulphate ions on flotation of galena. Miner. Eng. 2012, 39, 77–88. [Google Scholar] [CrossRef]
- Corin, K.C.; Wiese, J.G. Investigating froth stability: A comparative study of ionic strength and frother dosage. Miner. Eng. 2014, 66, 130–134. [Google Scholar] [CrossRef]
- Corin, K.C.; Reddy, A.; Miyen, L.; Wiese, J.G.; Harris, P.J. The effect of ionic strength of plant water on valuable mineral and gangue recovery in a platinum bearing ore from the Merensky reef. Miner. Eng. 2011, 24, 131–137. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C.; Wiese, J.G. An investigation into the effect of various ions and their ionic strength on the flotation performance of a platinum bearing ore from the Merensky reef. Miner. Eng. 2012, 36–38. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. Water quality effects on a sulfidic PGM ore: Implications for froth stability and gangue management. Physicochem. Probl. Miner. Process. 2018, 54, 1253–1265. [Google Scholar] [CrossRef]
- Wiese, J.; Harris, P.; Bradshaw, D. The influence of the reagent suite on the flotation of ores from the Merensky reef. Miner. Eng. 2005, 18, 189–198. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Harris, P.J.; O’Connor, C.T. Synergistic interactions between reagents in sulphide flotation. J. S. Afr. Inst. Min. Metall. 1998, 98, 189–194. [Google Scholar]
- Nyabeze, W.; McFadzean, B. Adsorption of copper sulphate on PGM-bearing ores and its influence on froth stability and flotation kinetics. Miner. Eng. 2016, 92, 28–36. [Google Scholar] [CrossRef]
- Mhlanga, S.S.; O’Connor, C.T.; McFadzean, B. A study of the relative adsorption of guar onto pure minerals. Miner. Eng. 2012, 36–38, 172–178. [Google Scholar] [CrossRef]
- du Bois, M.; Giles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Liu, Q.; O’Connor, C.T. Current understanding of the mechanism of polysaccharide adsorption at the mineral/aqueous solution interface. Int. J. Miner. Process. 2007, 84, 59–68. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Zanin, M. An investigation into the effect of water quality on froth stability. Adv. Powder Technol. 2012, 23, 493–497. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. Perspectives from literature on the influence of inorganic electrolytes present in plant water on flotation performance. Physicochem. Probl. Miner. Process. 2018, 54, 1191–1214. [Google Scholar] [CrossRef]
- Quinn, J.J.; Kracht, W.; Gomez, C.O.; Gagnon, C.; Finch, J.A. Comparing the effect of salts and frother (MIBC) on gas dispersion and froth properties. Miner. Eng. 2007, 20, 1296–1302. [Google Scholar] [CrossRef]
- Quinn, J.J.; Sovechles, J.M.; Finch, J.A.; Waters, K.E. Critical coalescence concentration of inorganic salt solutions. Miner. Eng. 2014, 58, 1–6. [Google Scholar] [CrossRef]
- Castro, S.; Miranda, C.; Toledo, P.; Laskowski, J.S. Effect of frothers on bubble coalescence and foaming in electrolyte solutions and seawater. Int. J. Miner. Process. 2013, 124, 8–14. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Cho, Y.S.; Ding, K. The effect of frothers on bubble size and foam stability in potash ore flotation systems. Can. J. Chem. Eng. 2003, 81, 63–69. [Google Scholar] [CrossRef]
- Ramos, O.; Castro, S.; Laskowski, J.S. Copper-molybdenum ores flotation in sea water: Floatability and frothability. Miner. Eng. 2013, 53, 108–112. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C.; Wiese, J.G. The effect of ionic strength of plant water on foam stability: A 2-phase flotation study. Miner. Eng. 2013, 40, 57–67. [Google Scholar] [CrossRef]

















| Water Type | Ca2+ (ppm) | Mg2+ (ppm) | Na+ (ppm) | Cl− (ppm) | SO42− (ppm) | NO3− (ppm) | CO32− (ppm) | TDS (mg/L) | IS [M] |
|---|---|---|---|---|---|---|---|---|---|
| 1SPW | 80 | 70 | 153 | 287 | 240 | 176 | 17 | 1023 | 0.0242 |
| 5SPW | 400 | 350 | 765 | 1435 | 1200 | 880 | 85 | 5115 | 0.1212 |
| 10SPW | 800 | 700 | 1530 | 2870 | 2400 | 1760 | 850 | 10,230 | 0.2426 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manono, M.; Corin, K.; Wiese, J. The Effect of the Ionic Strength of Process Water on the Interaction of Talc and CMC: Implications of Recirculated Water on Floatable Gangue Depression. Minerals 2019, 9, 231. https://doi.org/10.3390/min9040231
Manono M, Corin K, Wiese J. The Effect of the Ionic Strength of Process Water on the Interaction of Talc and CMC: Implications of Recirculated Water on Floatable Gangue Depression. Minerals. 2019; 9(4):231. https://doi.org/10.3390/min9040231
Chicago/Turabian StyleManono, Malibongwe, Kirsten Corin, and Jenny Wiese. 2019. "The Effect of the Ionic Strength of Process Water on the Interaction of Talc and CMC: Implications of Recirculated Water on Floatable Gangue Depression" Minerals 9, no. 4: 231. https://doi.org/10.3390/min9040231
APA StyleManono, M., Corin, K., & Wiese, J. (2019). The Effect of the Ionic Strength of Process Water on the Interaction of Talc and CMC: Implications of Recirculated Water on Floatable Gangue Depression. Minerals, 9(4), 231. https://doi.org/10.3390/min9040231