Effect of High Mixing Intensity on Rheological Properties of Cemented Paste Backfill
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Tailings
2.1.2. Cement
2.2. Mixture Contents
2.3. Preparation of Samples
2.4. Experimental Methods
2.4.1. Inductively Coupled Plasma Mass Spectrometry
2.4.2. Rheology
3. Results and Discussion
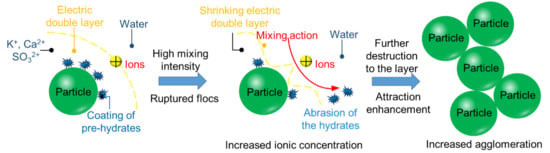
3.1. The Chemical Environment Changes of CPB
3.2. Rheological Properties
3.3. Thixotropic Breakdown
3.4. Structural Breakdown
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pileggi, R.G.; Studart, A.R.; Pandolfelli, V.C.; Gallo, J. How mixing affects the rheology of refractory castables—Part 2. Am. Ceram. Soc. Bull. 2001, 80, 38–42. [Google Scholar]
- Wu, A.; Ruan, Z.; Wang, Y.; Yin, S.; Wang, S.; Wang, Y.; Wang, J. Simulation of long-distance pipeline transportation properties of whole-tailings paste with high sliming. J. Cent. South Univ. 2018, 25, 141–150. [Google Scholar] [CrossRef]
- Fall, M.; Adrien, D.; Célestin, J.; Pokharel, M.; Touré, M. Saturated hydraulic conductivity of cemented paste backfill. Miner. Eng. 2009, 22, 1307–1317. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Ocak, S.; Akyol, Y. Effect of sodium-silicate activated slag at different silicate modulus on the strength and microstructural properties of full and coarse sulphidic tailings paste backfill. Constr. Build. Mater. 2018, 185, 555–566. [Google Scholar] [CrossRef]
- Wang, Y.; Fall, M.; Wu, A. Initial temperature-dependence of strength development and self-desiccation in cemented paste backfill that contains sodium silicate. Cem. Concr. Compos. 2016, 67, 101–110. [Google Scholar] [CrossRef]
- Mahlaba, J.; Kearsley, E.; Kruger, R.; Pretorius, P. Evaluation of workability and strength development of fly ash pastes prepared with industrial brines rich in SO4− and Cl− to expand brine utilization. Miner. Eng. 2011, 10, 1077–1081. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Wu, A.; Xing, P.; Gao, W. Thixotropy of unclassified pastes in the process of stirring and shearing. J. Univ. Sci. Technol. Beijing 2016, 38, 1343–1349. [Google Scholar]
- Zhang, Q.; Wang, X. Performance of cemented coal gangue backfill. J. Cent. South Univ. 2007, 14, 216–219. [Google Scholar] [CrossRef]
- Fall, M.; Célestin, J.; Pokharel, M.; Touré, M. A contribution to understanding the effect of temperatures on the mechanical properties of mine cemented tailings backfill: Experimental results. Eng. Geol. 2010, 114, 397–413. [Google Scholar] [CrossRef]
- Wu, D.; Cai, S. Coupled effect of cement hydration and temperature on hydraulic behavior of cemented tailings backfill. J. Cent. South Univ. 2015, 22, 1956–1964. [Google Scholar] [CrossRef]
- Baroud, G.; Samara, M.; Steffen, T. Influence of mixing method on the cement temperature-mixing time history and doughing time of three acrylic cements for vertebroplasty. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 68, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wendling, A.; Mar, D.; Wischmeier, N.; Anderson, D.; Mciff, T. Combination of modified mixing technique and low frequency ultrasound to control the elution profile of vancomycin-loaded acrylic bone cement. Bone Jt. Res. 2016, 5, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Li, X.; Yin, Z. A new manufacture method of backfill samples in lab—Illustrated with a case study. J. Cent. South Univ. 2013, 20, 1022–1028. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Saa, E. Mix proportioning of underground cemented tailings backfill. Tunn. Undergr. Space Technol. Inc. Trenchless Technol. Res. 2008, 23, 80–90. [Google Scholar] [CrossRef]
- Ferron, R.; Shah, S.; Fuente, E.; Negro, C. Aggregation and breakage kinetics of fresh cement paste. Cem. Concr. Res. 2013, 50. [Google Scholar] [CrossRef]
- Toutou, Z.; Roussel, N. Multi scale experimental study of concrete rheology: From water scale to gravel scale. Mater. Struct. 2006, 39, 189–199. [Google Scholar] [CrossRef]
- Jiang, H.; Fall, M. Yield stress and strength of saline cemented tailings materials in sub-zero environments: Slag-paste backfill. Int. J. Miner. Process. 2017, 160, 68–75. [Google Scholar] [CrossRef]
- Ghirian, A.; Fall, M. Paste Tailings Management; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 22–45. [Google Scholar]
- Wallevik, O.; Feys, D.; Wallevik, J.; Khayat, K. Avoiding inaccurate interpretations of rheological measurements for cement-based materials. Cem. Concr. Res. 2015, 78, 100–109. [Google Scholar] [CrossRef]
- Wallevik, J. Rheological properties of cement paste: Thixotropic behavior and structural breakdown. Cem. Concr. Res. 2009, 39, 14–29. [Google Scholar] [CrossRef]
- Wallevik, J. Particle Flow Interaction Theory-Thixotropic Behavior and Structural Breakdown. In Proceedings of the Conference on Our World of Concrete and Structures, Singapore, 14–16 August 2011; pp. 1–6. [Google Scholar]
- Hattori, K.; Izumi, K. Rheology of Fresh Cement and Concrete. In Rheology of Fresh Cement and Concrete, Proceedings of the International Conference, London, UK; CRC Press: Boca Raton, FL, USA, 1991; pp. 83–92. [Google Scholar]
- Hattori, K.; Izumi, K. A rheological expression of coagulation rate theory. J. Dispers. Sci. Technol. 1982, 3, 129–193. [Google Scholar] [CrossRef]
- Tattersall, G. The rheology of Portland cement pastes. Br. J. Appl. Phys. 1955, 6, 165–167. [Google Scholar] [CrossRef]
- Ritchie, A. The Rheology of Fresh Concrete; Pitman Books Limited: Boston, MA, USA, 1983; pp. 73–95. [Google Scholar]
- Wallevik, J. Rheology of Particle Suspensions: Fresh Concrete, Mortar and Cement Paste with Various Types of Lignosulfonates. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2003. [Google Scholar]
- Lapasin, R.; Papo, A.; Rajgelj, S. Flow behavior of fresh cement pastes. A comparison of different rheological instruments and techniques. Cem. Concr. Res. 1983, 13, 349–356. [Google Scholar] [CrossRef]
- Hunter, R.J. Foundations of Colloid Science, 2nd ed.; Oxford University Press: New York, NY, USA, 2001; pp. 101–132, 131–144. [Google Scholar]
- Williams, D.; Saak, A.; Jennings, H. The influence of mixing on the rheology of fresh cement paste. Cem. Concr. Res. 1999, 29, 1491–1496. [Google Scholar] [CrossRef]
- Tattersall, G. Structural breakdown of cement pastes at constant rate of shear. Nature 1955, 175, 166. [Google Scholar] [CrossRef]
- Ahari, R.; Erdem, T.; Ramyar, K. Thixotropy and structural breakdown properties of self-consolidating concrete containing various supplementary cementitious materials. Cem. Concr. Compos. 2015, 59, 26–37. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.; Livingston, R.; Nonat, A.; Scherer, G.; Schweitzer, J.; Scrivener, K.; Thoma, J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Takahashi, K.; Bier, T.A. Westphal. Effects of mixing energy on technological properties and hydration kinetics of grouting mortars. Cem. Concr. Res. 2011, 41, 1167–1176. [Google Scholar] [CrossRef]
- Cazacliu, B. In-mixer measurements for describing mixture evolution during concrete mixing. Chem. Eng. Res. Des. 2008, 86, 1423–1433. [Google Scholar] [CrossRef]
- Cazacliu, B.; Roquet, N. Concrete mixing kinetics by means of power measurement. Cem. Concr. Res. 2009, 39, 182–194. [Google Scholar] [CrossRef]
- He, Z.; Xie, K.; Zhang, C.; Xie, C. Activating mixing technology and its application in mine backfill. GOLD 2000, 21, 18–20. [Google Scholar]
- Landriault, D.A. Backfill in underground mining. In Underground mining methods: Engineering Fundamentals and International Case Studies; Hustrulid, R.L., Bulloch, W., Eds.; Society for Mining, Metallurgy and Exploration-SME: Lilleton, CO, USA, 2001; pp. 601–614. [Google Scholar]
- Barnes, A.; Merseyside, L.; Carnali, O. The vane-in-cup as a novel rheometer geometry for shear thinning and thixotropic materials. J. Rheol. 1990, 34, 841–866. [Google Scholar] [CrossRef]
- Wu, D.; Fall, M.; Cai, S. Coupling temperature, cement hydration and rheological behaviour of fresh cemented paste backfill. Miner. Eng. 2013, 42, 76–87. [Google Scholar] [CrossRef]
- Locher, F.W. Zement-Grundlagen der Herstellung und Verwendung; Vbt Verlag Bau+ Technik: Düsseldorf, Germany, 2000. [Google Scholar]
- Kim, W.; Yang, S. Microstructures and Rheological Responses of Aqueous CTAB Solutions in the Presence of Benzyl Additives. Langmuir 2000, 16, 6084–6093. [Google Scholar] [CrossRef]
- Dintzis, F.; Berhow, M.; Bagley, E.; Wu, Y.; Felker, F. Shear-thickening behavior and shear-induced structure in gently solubilized starches. Cereal Chem. 1996, 73, 638–643. [Google Scholar]
- Roussel, N. A thixotropy model for fresh fluid concretes: Theory, validation and applications. Cem. Concr. Res. 2006, 36, 1797–1806. [Google Scholar] [CrossRef]
- Liu, X.; Wu, A.; Wang, H.; Jiao, H.; Liu, S.; Wang, S. Experimental Studies on the Thixotropic Characteristics of Unclassified-tailings Paste Slurry. J. Wuhan Univ. Technol. 2014, 38, 539–543. [Google Scholar]
- Rudman, M.; Blackburn, M.; Graham, J.; Pullum, L. Turbulent Pipe Flow of Shear-Thinning Fluids. J. Non-Newton. Fluid Mech. 2004, 118, 33–48. [Google Scholar] [CrossRef]
- Larson, R. Constitutive equations for thixotropic fluids. J. Rheol. 2015, 59, 595–611. [Google Scholar] [CrossRef] [Green Version]
- Barnes, H. Thixotropy—A review. Non-Newton. Fluid Mech. 1997, 70, 1–33. [Google Scholar] [CrossRef]
- Barnes, H.; Nguyen, Q. Rotating vane rheometry—A review. J. Non-Newton. Fluid Mech. 2001, 98, S0257–S0377. [Google Scholar] [CrossRef]
- Overbeek, J. Interparticle forces in colloid science. Powder Technol. 1984, 37, 195–208. [Google Scholar] [CrossRef]
- Han, D.; Ferron, R. Influence of high mixing intensity on rheology, hydration, and microstructure of fresh state cement paste. Cem. Concr. Res. 2016, 84, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Ferron, R. Set-on-demand concrete. Cem. Concr. Res. 2014, 57, 13–27. [Google Scholar] [CrossRef]
- Struble, L.; Lei, W. Rheological changes associated with setting of cement paste. Adv. Cem. Based Mater. 1995, 2, 224–230. [Google Scholar] [CrossRef]
- Erdem, K.; Khayat, K.; Yahia, A. Correlating, rheology of self-consolidating concrete to corresponding concrete-equivalent mortar. ACI Mater. J. 2010, 106, 154–160. [Google Scholar]
- Cosgrove, T. Colloid Science: Principles, Methods and Applications; Blackwell Publishing: Ames, IA, USA, 2005; pp. 76–95. [Google Scholar]
- Yang, M.; Neubauer, C.; Jennings, H. Interparticle potential and sedimentation behavior of cement suspensions-Review and results from paste. Adv. Cem. Based Mater. 1997, 5. [Google Scholar] [CrossRef]
- Ferron, R. Formwork Pressure of Self-Consolidating Concrete: Influence of Flocculation Mechanisms, Structural Rebuilding, Thixotropy and Rheology. Ph.D. Thesis, Northwestern University, Evanston, IL, USA, 2008. [Google Scholar]
- Takahashi, K.; Bier, T. Effects of mixing action on hydration kinetics and hardening properties of cement-based mortars. Cem. Sci. Concr. Technol. 2015, 69, 161–168. [Google Scholar] [CrossRef]
- Han, D.; Ferron, R. Effect of mixing method on microstructure and rheology of cement paste. Constr. Build. Mater. 2015, 93, 278–288. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Bire, A. Mechanisms of Degradation in Rheological Properties Due to Pumping and Mixing. Adv. Civ. Eng. Mater. 2014, 3, 25–39. [Google Scholar] [CrossRef]











| Compound | Pb | Zn | S | As | Au | Ag | CaO | MgO | Al2O3 | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Content/% | 0.035 | 0.003 | 0.39 | 0.057 | 0.03 | 1.59 | 9.16 | 1.4 | 6.19 | 64.69 |
| Items | MgO | SiO2 | Na2O | K2O | Al2O3 | SO3 | Fe2O3 | CaO |
|---|---|---|---|---|---|---|---|---|
| Amount (%) | 1.40 | 20.70 | 0.18 | 0.48 | 4.50 | 2.60 | 3.30 | 65.10 |
| Code | Water w/c (by mass) | Cement (wt %) | Tailings t/c (by mass) | Solids Content (wt %) | Solids Content (vol. %) |
|---|---|---|---|---|---|
| CPB-A | 2.75 | 8.12 | 8.56 | 77.66 | 56 |
| CPB-B | 2.75 | 8.64 | 7.83 | 76.25 | 54 |
| CPB-C | 2.75 | 9.17 | 7.16 | 74.79 | 52 |
| CPB-D | 2.25 | 11.17 | 5.70 | 74.86 | 52 |
| High-Shearing-Type Mixer (rpm) | High-Intensity Mixer (rpm) | Estimated Shear Rate (s−1) |
|---|---|---|
| 100 | 334.78 | 120.14 |
| 200 | 669.57 | 240.28 |
| 300 | 1004.35 | 360.42 |
| 400 | 1339.13 | 480.56 |
| 500 | 1673.91 | 600.70 |
| 600 | 2008.70 | 720.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wang, H.; Li, H.; Zhou, X. Effect of High Mixing Intensity on Rheological Properties of Cemented Paste Backfill. Minerals 2019, 9, 240. https://doi.org/10.3390/min9040240
Yang L, Wang H, Li H, Zhou X. Effect of High Mixing Intensity on Rheological Properties of Cemented Paste Backfill. Minerals. 2019; 9(4):240. https://doi.org/10.3390/min9040240
Chicago/Turabian StyleYang, Liuhua, Hongjiang Wang, Hong Li, and Xu Zhou. 2019. "Effect of High Mixing Intensity on Rheological Properties of Cemented Paste Backfill" Minerals 9, no. 4: 240. https://doi.org/10.3390/min9040240
APA StyleYang, L., Wang, H., Li, H., & Zhou, X. (2019). Effect of High Mixing Intensity on Rheological Properties of Cemented Paste Backfill. Minerals, 9(4), 240. https://doi.org/10.3390/min9040240