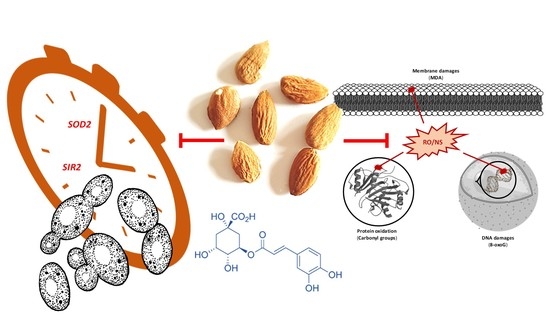
Almond Skin Extracts and Chlorogenic Acid Delay Chronological Aging and Enhanced Oxidative Stress Response in Yeast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extract Preparation
2.3. HPLC Analysis
2.4. Yeast Culture Conditions
2.5. Reactive Oxygen and Nitrogen Species Measurement
2.6. Estimation of NAD and NADH Contents
2.7. Mitochondria Membrane Potential Evaluation
2.8. Gene Expression by RT-qPCR Analysis
2.9. Enzymatic SIRT1/SIR2 and Total SOD Activities Determinations
2.10. UV-Induced Oxidative Stress and Survival (Cell Viability) to Oxidative Stress Evaluation
2.11. Membrane Lipid Peroxidation Evaluation
2.12. Protein Carbonylation Level Estimation
2.13. 8-oxo-guanine Level Estimation
2.14. Statistical Analysis
3. Results and Discussion
3.1. Yeast Lifespan Extension induced by Almond Skin Extract Chlorogenic Acid is Accompanied by a Reduction in Reactive Oxygen/Nitrogen Species
3.2. Almond Skin Extract and Chlorogenic Acid Activated Expression of Genes Involved in Oxidative Stress Resistance
3.3. Almond Skin Extract and Chlorogenic Acid Increased Yeast Survival to Oxidative Stress induced by UV-C and Reduced Oxidative Cell Damages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–919. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W. Almond polyphenols: Methods of analysis, contribution to food quality, and health promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef] [Green Version]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I. Valorization challenges to almond residues: Phytochemical composition and functional application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [Green Version]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I. A Box-Behnken Design for Optimal Extraction of Phenolics from Almond By-products. Food Anal. Methods 2019, 12, 2009–2024. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Elamrani, A.; Abid, M.; Drouet, S.; Kiani, R.; Garros, L.; Kabra, A.; Addi, M.; Hano, C. A Quick, Green and Simple Ultrasound-Assisted Extraction for the Valorization of Antioxidant Phenolic Acids from Moroccan Almond (Prunus dulcis (Mill.) DA Webb) Cold-Pressed Oil Residues. Appl. Sci. 2020, 10, 3313. [Google Scholar] [CrossRef]
- Delplancke, M.; Aumeeruddy-thomas, Y. Des semis et des clones. Rev. Ethnoécol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Melhaoui, R.; Fauconnier, M.-L.; Sindic, M.; Addi, M.; Abid, M.; Mihamou, A.; Serghini-Caid, H.; Elamrani, A. Tocopherol content of almond oils produced in eastern Morocco. In Proceedings of the 23rd National Symposium for Applied Biological Sciences (NSABS), Brussels, Belgium, 8 February 2018; pp. 75–77. [Google Scholar]
- Pomatto, L.C.D.; Davies, K.J.A. Adaptive homeostasis and the free radical theory of ageing. Free Radic. Biol. Med. 2018, 124, 420–430. [Google Scholar] [CrossRef]
- Harman, D. The free radical theory of aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radical chemistry. J Gerontol. 1956, 11, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.P.; Sinclair, D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014, 35, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Zimmermann, A.; Hofer, S.; Pendl, T.; Kainz, K.; Madeo, F.; Carmona-Gutierrez, D. Yeast as a tool to identify anti-aging compounds. FEMS Yeast Res. 2018, 18, foy020. [Google Scholar] [CrossRef]
- He, C.; Zhou, C.; Kennedy, B.K. The yeast replicative aging model. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2690–2696. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Sun, K.; Lu, J.; Weng, Y.; Taoka, A.; Sakagami, Y.; Qi, J. Anti-aging effects of phloridzin, an apple polyphenol, on yeast via the SOD and Sir2 genes. Biosci. Biotechnol. Biochem. 2011, 75, 854–858. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Xiang, L.; Ishihara, S.; Matsuura, A.; Sakagami, Y.; Qi, J. Anti-aging effects of hesperidin on Saccharomyces cerevisiae via inhibition of reactive oxygen species and UTH1 gene expression. Biosci. Biotechnol. Biochem. 2012, 1202232809. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Cao, S.; Pei, L.; Matsuura, A.; Xiang, L.; Qi, J. A steroidal saponin from Ophiopogon japonicus extends the lifespan of yeast via the pathway involved in SOD and UTH1. Int. J. Mol. Sci. 2013, 14, 4461–4475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierman, M.B.; Smith, J.S. Yeast sirtuins and the regulation of aging. FEMS Yeast Res. 2014, 14, 73–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaeberlein, M.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merksamer, P.I.; Liu, Y.; He, W.; Hirschey, M.D.; Chen, D.; Verdin, E. The sirtuins, oxidative stress and aging: An emerging link. Aging 2013, 5, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Wei, M.; Mirisola, M.G.; Longo, V.D. Assessing chronological aging in Saccharomyces cerevisiae. In Cell Senescence. Methods in Molecular Biology (Methods and Protocols); Galluzzi, L., Vitale, I., Kepp, O., Kroemer, G., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 965, pp. 463–472. [Google Scholar]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; De Lacour, J.L.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the influence of cultivar type, cultivation year, and site on the lignans and related phenolic profiles, and the health-promoting antioxidant potential of flax (linum usitatissimum L.) seeds. Molecules 2018, 23, 2636. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’h, N.; Abbasi, B.H.; Hano, C. Differential Production of Phenylpropanoid Metabolites in Callus Cultures of Ocimum basilicum L. With Distinct in Vitro Antioxidant Activities and in Vivo Protective Effects against UV stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef]
- Lin, S.S.; Manchester, J.K.; Gordon, J.I. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 36000–36007. [Google Scholar] [CrossRef] [Green Version]
- Hano, C.; Addi, M.; Fliniaux, O.; Bensaddek, L.; Duverger, E.; Mesnard, F.; Lamblin, F.; Lainé, E. Molecular characterization of cell death induced by a compatible interaction between Fusarium oxysporum f. sp. linii and flax (Linum usitatissimum) cells. Plant Physiol. Biochem. 2008, 46, 590–600. [Google Scholar] [CrossRef]
- Petit, P.X.; O’Connor, J.E.; Grunwald, D.; Brown, S.C. Analysis of the membrane potential of rat-and mouse-liver mitochondria by flow cytometry and possible applications. Eur. J. Biochem. 1990, 194, 389–397. [Google Scholar] [CrossRef]
- Fabrizio, P.; Longo, V.D. The chronological life span of Saccharomyces cerevisiae. Aging Cell 2003, 2, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Xiang, L.; Matsuura, A.; Zhang, Y.; Huang, Q.; Qi, J. Ganodermasides A and B, two novel anti-aging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorg. Med. Chem. 2010, 18, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, Y.; Cao, X.; Xiang, L.; Qi, J. Sterols from Mytilidae show anti-aging and neuroprotective effects via anti-oxidative activity. Int. J. Mol. Sci. 2014, 15, 21660–21673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, U.; Pan, Y.; Lin, Y.; Wang, Y.; Osada, H.; Xiang, L.; Qi, J. Structure Characterization and Action Mechanism of an Antiaging New Compound from Gastrodia elata Blume. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Sun, Y.; Lin, Y.; Pan, Y.; Farooq, U.; Xiang, L.; Qi, J. Antiaging of Cucurbitane Glycosides from Fruits of Momordica charantia L. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Spindler, S.R.; Mote, P.L.; Flegal, J.M. Lifespan effects of simple and complex nutraceutical combinations fed isocalorically to mice. Age (Omaha) 2014, 36, 705–718. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Watts, K.T.; Lee, P.C.; Schmidt-Dannert, C. Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol. 2006, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Halls, C.; Zhang, J.; Matsuno, M.; Zhang, Y.; Yu, O. Stepwise increase of resveratrol biosynthesis in yeast Saccharomyces cerevisiae by metabolic engineering. Metab. Eng. 2011, 13, 455–463. [Google Scholar] [CrossRef]
- Jordan, P.; Choe, J.-Y.; Boles, E.; Oreb, M. Hxt13, Hxt15, Hxt16 and Hxt17 from Saccharomyces cerevisiae represent a novel type of polyol transporters. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Pen, G.; Long, S.; Yang, C. Yeast-cell-based microencapsulation of chlorogenic acid as a water-soluble antioxidant. J. Food Eng. 2007, 80, 1060–1067. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Int. J. Pharm. 2008, 349, 83–93. [Google Scholar] [CrossRef]
- Lacza, Z.; Pankotai, E.; Csordás, A.; Gero, D.; Kiss, L.; Horváth, E.M.; Kollai, M.; Busija, D.W.; Szabó, C. Mitochondrial NO and reactive nitrogen species production: Does mtNOS exist? Nitric Oxide 2006, 14, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-J.; Ford, E.; Haigis, M.; Liszt, G.; Guarente, L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004, 18, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, V.; Mattivi, F.; Silvestri, R.; La Regina, G.; Falcone, C.; Mazzoni, C. Apple can act as anti-aging on yeast cells. Oxid. Med. Cell. Longev. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanno, M.; Kuno, A.; Yano, T.; Miura, T.; Hisahara, S.; Ishikawa, S.; Shimamoto, K.; Horio, Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010, 285, 8375–8382. [Google Scholar] [CrossRef] [Green Version]
- Semchyshyn, H.M.; Lozinska, L.M. Fructose protects baker’s yeast against peroxide stress: Potential role of catalase and superoxide dismutase. FEMS Yeast Res. 2012, 12, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Fabrizio, P.; Pletcher, S.D.; Minois, N.; Vaupel, J.W.; Longo, V.D. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 2004, 557, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Daitoku, H.; Hatta, M.; Matsuzaki, H.; Aratani, S.; Ohshima, T.; Miyagishi, M.; Nakajima, T.; Fukamizu, A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10042–10047. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.-Q.; Huang, X.-B.; Xing, T.-K.; Ding, A.-J.; Wu, G.-S.; Luo, H.-R. Chlorogenic acid extends the lifespan of Caenorhabditis elegans via insulin/IGF-1 signaling pathway. J. Gerontol. Ser. A 2017, 72, 464–472. [Google Scholar]
- Kato, M.; Ochiai, R.; Kozuma, K.; Sato, H.; Katsuragi, Y. Effect of chlorogenic acid intake on cognitive function in the elderly: A pilot study. Evid. Based Complement. Altern. Med. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, K.; Green, E.M. Assessing Yeast Cell Survival Following Hydrogen Peroxide Exposure. Bio Protoc. 2019, 9, e3149. [Google Scholar] [CrossRef] [PubMed]
- De Alves, G.A.D.; de Souza, R.O.; Rogez, H.L.G.; Masaki, H.; Fonseca, M.J.V. Cecropia obtusa extract and chlorogenic acid exhibit anti aging effect in human fibroblasts and keratinocytes cells exposed to UV radiation. PLoS ONE 2019, 14, e0216501. [Google Scholar] [CrossRef] [PubMed]
- Kanvah, S.; Joseph, J. amd RN, Barnett GB, Schuster, Cleveland CL, Landman U. Acc. Chem. Res. 2010, 43, 280. [Google Scholar] [CrossRef]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G-T and A-C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungmunnithum, D.; Abid, M.; Elamrani, A.; Drouet, S.; Addi, M.; Hano, C. Almond Skin Extracts and Chlorogenic Acid Delay Chronological Aging and Enhanced Oxidative Stress Response in Yeast. Life 2020, 10, 80. https://doi.org/10.3390/life10060080
Tungmunnithum D, Abid M, Elamrani A, Drouet S, Addi M, Hano C. Almond Skin Extracts and Chlorogenic Acid Delay Chronological Aging and Enhanced Oxidative Stress Response in Yeast. Life. 2020; 10(6):80. https://doi.org/10.3390/life10060080
Chicago/Turabian StyleTungmunnithum, Duangjai, Malika Abid, Ahmed Elamrani, Samantha Drouet, Mohamed Addi, and Christophe Hano. 2020. "Almond Skin Extracts and Chlorogenic Acid Delay Chronological Aging and Enhanced Oxidative Stress Response in Yeast" Life 10, no. 6: 80. https://doi.org/10.3390/life10060080
APA StyleTungmunnithum, D., Abid, M., Elamrani, A., Drouet, S., Addi, M., & Hano, C. (2020). Almond Skin Extracts and Chlorogenic Acid Delay Chronological Aging and Enhanced Oxidative Stress Response in Yeast. Life, 10(6), 80. https://doi.org/10.3390/life10060080