Active Biomolecules from Vegetable Extracts with Antitumoral Activity against Pancreas Cancer: A Systematic Review (2011–2021)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Eligibility
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Sources
2.5. Study Selection
2.6. Data Extraction
3. Results
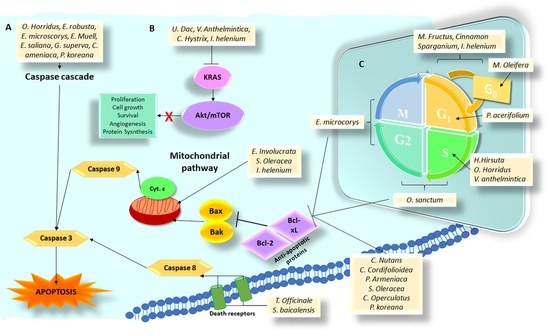
3.1. Plant Species and Isolated Compounds That Induce Cell Death by Apoptosis
3.2. Plant Species and Isolated Compounds That Induce Cell Death through Alteration of Pathways Activated by KRAS Mutation
3.3. Plant Species and Isolated Compounds That Induce Cell Death through Arrest in Some Phase of the Cell Cycle
3.4. Plant Species and Isolated Compounds That Induce Cell Death through the Alteration of Other Important Factors in the Formation of Pancreas Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari, D.; Tingstedt, B.; Andersson, B.; Holmquist, F.; Sturesson, C.; Williamsson, C.; Sasor, A.; Borg, D.; Bauden, M.; Andersson, R. Pancreatic Cancer: Yesterday, Today and Tomorrow. Future Oncol. 2016, 12, 1929–1946. [Google Scholar] [CrossRef] [Green Version]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic Cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Stan, S.D.; Singh, S.V.; Brand, R.E. Chemoprevention Strategies for Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 347–356. [Google Scholar] [CrossRef]
- De Souza, P.G.; Rosenthal, A.; Ayres, E.M.M.; Teodoro, A.J. Potential Functional Food Products and Molecular Mechanisms of Portulaca oleracea L. on Anticancer Activity: A Review. Oxid Med. Cell. Longev. 2022, 2022, 7235412. [Google Scholar] [CrossRef]
- Li, L.; Leung, P.S. Use of Herbal Medicines and Natural Products: An Alternative Approach to Overcoming the Apoptotic Resistance of Pancreatic Cancer. Int. J. Biochem. Cell Biol. 2014, 53, 224–236. [Google Scholar] [CrossRef]
- Deng, L.J.; Qi, M.; Li, N.; Lei, Y.H.; Zhang, D.M.; Chen, J.X. Natural Products and Their Derivatives: Promising Modulators of Tumor Immunotherapy. J. Leukoc. Biol. 2020, 108, 493–508. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Fazmiya, M.J.A.; Sultana, A.; Rahman, K.; Bin Heyat, M.B.; Sumbul; Akhtar, F.; Khan, S.; Appiah, S.C.Y. Current Insights on Bioactive Molecules, Antioxidant, Anti-Inflammatory, and Other Pharmacological Activities of Cinnamomum camphora Linn. Oxid Med. Cell. Longev. 2022, 2022, 9354555. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, N.; Liu, Y.; Zhang, H.; Yang, Y.; Liu, L.; Feng, J. Bioactive Compounds from Medicinal Mushrooms. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–50. [Google Scholar] [CrossRef]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Alamoudi, S.A.; Saad, A.M.; Alsubhi, N.H.; Alrefaei, G.I.; Al-Quwaie, D.A.; Binothman, N.; Aljadani, M.; Alharbi, M.; Alanazi, H.; Babalghith, A.O.; et al. Upgrading the Physiochemical and Sensory Quality of Yogurt by Incorporating Polyphenol-Enriched Citrus Pomaces with Antioxidant, Antimicrobial, and Antitumor Activities. Front. Nutr. 2022, 9, 999581. [Google Scholar] [CrossRef]
- Menger, L.; Vacchelli, E.; Kepp, O.; Eggermont, A.; Tartour, E.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Cardiac Glycosides and Cancer Therapy. Oncoimmunology 2013, 2, e23082. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.J.; Ho, C.C.; Ho, H.; Chen, W.J.; Lin, C.H.; Lai, Y.H.; Juan, Y.C.; Chu, W.C.; Lee, J.H.; Su, S.F.; et al. Tumor Microenvironment-Based Screening Repurposes Drugs Targeting Cancer Stem Cells and Cancer-Associated Fibroblasts. Theranostics 2021, 11, 9667–9686. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Hou, L.; Wang, W.; Li, K.; Zhang, Z.; Du, B.; Kong, D. Digoxin Exerts Anticancer Activity on Human Nonsmall Cell Lung Cancer Cells by Blocking PI3K/Akt Pathway. Biosci. Rep. 2021, 41, BSR20211056. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, S.; Burdette, J.E.; Cheng, X.; Kinghorn, A.D. Structural Insights into the Interactions of Digoxin and Na+/K+-ATPase and Other Targets for the Inhibition of Cancer Cell Proliferation. Molecules 2021, 26, 3672. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, A.; Zhang, S.; Kim, J.; Xia, J.; Zhang, F.; Wang, D.; Wang, Q.; Wang, J. Paclitaxel-Loaded Ginsenoside Rg3 Liposomes for Drug-Resistant Cancer Therapy by Dual Targeting of the Tumor Microenvironment and Cancer Cells. J. Adv. Res. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, S.; Zhang, X.; Jin, C.; Zhao, B.; Li, L.; Miao, Q.R.; Jin, Y.; Fan, Z. Nogo-B Receptor Increases Glycolysis and the Paclitaxel Resistance of Estrogen Receptor-Positive Breast Cancer via the HIF-1α-Dependent Pathway. Cancer Gene Ther. 2022; in press. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, F.; Zhao, Y.; Li, P.; Wang, T.; Xu, Z.; Zhang, J.; Zhang, W. A Novel Ligand-Modified Nanocomposite Microparticles Improved Efficiency of Quercetin and Paclitaxel Delivery in the Non-Small Cell Lung Cancer. Drug Deliv. 2022, 29, 3123–3133. [Google Scholar] [CrossRef]
- Chen, J.L.; Dawoodji, A.; Tarlton, A.; Gnjatic, S.; Tajar, A.; Karydis, I.; Browning, J.; Pratap, S.; Verfaille, C.; Venhaus, R.R.; et al. NY-ESO-1 Specific Antibody and Cellular Responses in Melanoma Patients Primed with NY-ESO-1 Protein in ISCOMATRIX and Boosted with Recombinant NY-ESO-1 Fowlpox Virus. Int. J. Cancer 2015, 136, E590–E601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hong, J.A.; Kunst, T.F.; Bond, C.D.; Kenney, C.M.; Warga, C.L.; Yeray, J.; Lee, M.J.; Yuno, A.; Lee, S.; et al. Randomized Phase II Trial of a First-in-Human Cancer Cell Lysate Vaccine in Patients with Thoracic Malignancies. Transl. Lung Cancer Res. 2021, 10, 3079–3092. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-Step Guide on How to Design, Conduct, and Successfully Publish a Systematic Review and Meta-Analysis in Medical Research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Száva-Kováts, E. Unfounded Attribution of the “Half-Life” Index-Number of Literature Obsolescence to Burton and Kebler: A Literature Science Study. J. Am. Soc. Inf. Sci. Technol. 2002, 53, 1098–1105. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Sanz-Valero, J. Systematic Reviews in Nutrition: Standardized Methodology. Br. J. Nutr. 2012, 107, S3–S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Multiple Regression as a General Data-Analytic System. Psychol. Bull. 1968, 70, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Cheung, S.S.C.; Hasman, D.; Khelifi, D.; Tai, J.; Smith, R.W.; Warnock, G.L. Devil’s Club Falcarinol-Type Polyacetylenes Inhibit Pancreatic Cancer Cell Proliferation. Nutr. Cancer 2019, 71, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.S.C.; Tai, J.; Hasman, D.; Ou, D.; Warnock, G.L. Inhibition of Human Pancreatic Cancer Cell Proliferation by Devil’s Club Oplopanax Horridus and Its Polyacetylene Bioactive Compound. Nutr. Cancer 2015, 67, 954–964. [Google Scholar] [CrossRef]
- Girardelo, J.R.; Munari, E.L.; Dallorsoleta, J.C.S.; Cechinel, G.; Goetten, A.L.F.; Sales, L.R.; Reginatto, F.H.; Chaves, V.C.; Smaniotto, F.A.; Somacal, S.; et al. Bioactive Compounds, Antioxidant Capacity and Antitumoral Activity of Ethanolic Extracts from Fruits and Seeds of Eugenia Involucrata DC. Food Res. Int. 2020, 137, 109615. [Google Scholar] [CrossRef]
- Tuan, H.N.; Minh, B.H.; Tran, P.T.; Lee, J.H.; van Oanh, H.; Thi Ngo, Q.M.; Nguyen, Y.N.; Kim Lien, P.T.; Tran, M.H. The Effects of 20,40-Dihydroxy-60-Methoxy-30,50-Dimethylchalcone from Cleistocalyx Operculatus Buds on Human Pancreatic Cancer Cell Lines. Molecules 2019, 24, 2538. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, D.J.; Sakoff, J.; Bond, D.R.; Predebon, M.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. In Vitro Anticancer Properties of Selected Eucalyptus Species. In Vitro Cell Dev. Biol. Anim. 2017, 53, 604–615. [Google Scholar] [CrossRef]
- Capistrano, R.; Vangestel, C.; Wouters, A.; Dockx, Y.; Pauwels, P.; Stroobants, S.; Apers, S.; Lardon, F.; Pieters, L.; Staelens, S. Efficacy Screening of Gloriosa Superba Extracts in a Murine Pancreatic Cancer Model Using 18F-FDG PET/CT for Monitoring Treatment Response. Cancer Biother. Radiopharm. 2016, 31, 99–109. [Google Scholar] [CrossRef]
- Aamazadeh, F.; Ostadrahimi, A.; Rahbar Saadat, Y.; Barar, J. Bitter Apricot Ethanolic Extract Induces Apoptosis through Increasing Expression of Bax/Bcl-2 Ratio and Caspase-3 in PANC-1 Pancreatic Cancer Cells. Mol. Biol. Rep. 2020, 47, 1895–1904. [Google Scholar] [CrossRef]
- Hii, L.W.; Lim, S.H.E.; Leong, C.O.; Chin, S.Y.; Tan, N.P.; Lai, K.S.; Mai, C.W. The Synergism of Clinacanthus Nutans Lindau Extracts with Gemcitabine: Downregulation of Anti-Apoptotic Markers in Squamous Pancreatic Ductal Adenocarcinoma. BMC Complement. Altern. Med. 2019, 19, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, Y.P.; Li, Q.F.; Wu, S.G.; Mao, D.C.; Deng, Y.Y.; Chen, R.W. Tsoong Induces Apoptosis and Inhibits Proliferation, Migration and Invasion of Pancreatic Ductal Adenocarcinoma Cells. Mol. Med. Rep. 2018, 17, 3527–3536. [Google Scholar] [CrossRef] [Green Version]
- Akasaka, H.; Mizushina, Y.; Yoshida, K.; Ejima, Y.; Mukumoto, N.; Wang, T.; Inubushi, S.; Nakayama, M.; Wakahara, Y.; Sasaki, R. MGDG Extracted from Spinach Enhances the Cytotoxicity of Radiation in Pancreatic Cancer Cells. Radiat. Oncol. 2016, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, H.; Wang, J.; Chang, Q.; Hu, Z.; Shen, X.; Feng, J.; Zhang, Z.; Wu, X. Total Flavonoid Aglycones Extract in Radix Scutellariae Induces Cross-Regulation between Autophagy and Apoptosis in Pancreatic Cancer Cells. J. Ethnopharmacol. 2019, 235, 133–140. [Google Scholar] [CrossRef]
- Ovadje, P.; Chochkeh, M.; Akbari-Asl, P.; Hamm, C.; Pandey, S. Selective Induction of Apoptosis and Autophagy through Treatment with Dandelion Root Extract in Human Pancreatic Cancer Cells. Pancreas 2012, 41, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.Y.; Athikomkulchai, S.; Miyatake, R.; Saiki, I.; Esumi, H.; Awale, S. (+)-Grandiforacin, an Antiausterity Agent, Induces Autophagic PANC-1 Pancreatic Cancer Cell Death. Drug Des. Dev. Ther. 2013, 8, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Quan, Q.; Ji, R.; Guo, X.Y.; Zhang, J.M.; Li, X.; Liu, Y.G. Isorhamnetin Suppresses PANC-1 Pancreatic Cancer Cell Proliferation through S Phase Arrest. Biomed. Pharmacother. 2018, 108, 925–933. [Google Scholar] [CrossRef]
- Sun, S.; Phrutivorapongkul, A.; Dibwe, D.F.; Balachandran, C.; Awale, S. Chemical Constituents of Thai Citrus Hystrix and Their Antiausterity Activity against the PANC-1 Human Pancreatic Cancer Cell Line. J. Nat. Prod. 2018, 81, 1877–1883. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, J.; Yan, Y.; Yang, B.; Huang, M.; Wang, L.; Zhang, Q.; Lin, N. Ethyl Acetate Extract from Inula Helenium L. Inhibits the Proliferation of Pancreatic Cancer Cells by Regulating the STAT3/AKT Pathway. Mol. Med. Rep. 2018, 17, 5440–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, H.N.T.; Van Vuong, Q.; Bowyer, M.C.; Scarlett, C.J. In Vitro Anti-Pancreatic Cancer Activity of HPLC-Derived Fractions from Helicteres Hirsuta Lour. Stem. Mol. Biol. Rep. 2020, 47, 897–905. [Google Scholar] [CrossRef]
- Shimizu, T.; Torres, M.P.; Chakraborty, S.; Souchek, J.J.; Rachagani, S.; Kaur, S.; Macha, M.; Ganti, A.K.; Hauke, R.J.; Batra, S.K. Holy Basil Leaf Extract Decreases Tumorigenicity and Metastasis of Aggressive Human Pancreatic Cancer Cells in Vitro and in Vivo: Potential Role in Therapy. Cancer Lett. 2013, 336, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, S.K.; Biswal, B.K. Pterospermum acerifolium (L.) Wild Bark Extract Induces Anticarcinogenic Effect in Human Cancer Cells through Mitochondrial-Mediated ROS Generation. Mol. Biol. Rep. 2018, 45, 2283–2294. [Google Scholar] [CrossRef]
- Pak, P.J.; Kang, B.H.; Park, S.H.; Sung, J.H.; Joo, Y.H.; Jung, S.H.; Chung, N. Antitumor Effects of Herbal Mixture Extract in the Pancreatic Adenocarcinoma Cell Line PANC1. Oncol. Rep. 2016, 36, 2875–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, J.; Cheung, S.S.C.; Ou, D.; Warnock, G.L.; Hasman, D. Antiproliferation Activity of Devil’s Club (Oplopanax horridus) and Anticancer Agents on Human Pancreatic Cancer Multicellular Spheroids. Phytomedicine 2014, 21, 506–514. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Bond, D.R.; Chalmers, A.C.; Bowyer, M.C.; Scarlett, C.J. Eucalyptus Microcorys Leaf Extract Derived HPLC-Fraction Reduces the Viability of MIA PaCa-2 Cells by Inducing Apoptosis and Arresting Cell Cycle. Biomed. Pharmacother. 2018, 105, 449–460. [Google Scholar] [CrossRef]
- Berkovich, L.; Earon, G.; Ron, I.; Rimmon, A.; Vexler, A.; Lev-Ari, S. Moringa Oleifera Aqueous Leaf Extract Down-Regulates Nuclear Factor-KappaB and Increases Cytotoxic Effect of Chemotherapy in Pancreatic Cancer Cells. BMC Complement. Altern. Med. 2013, 13, 212. [Google Scholar] [CrossRef] [Green Version]
- Son, M.K.; Jung, K.H.; Lee, H.S.; Lee, H.; Kim, S.J.; Yan, H.H.; Ryu, Y.L.; Hong, S.S. SB365, Pulsatilla Saponin D Suppresses Proliferation and Induces Apoptosis of Pancreatic Cancer Cells. Oncol. Rep. 2013, 30, 801–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.S.; Kim, M.K.; Lee, K.; Lee, K.M.; Choi, Y.K.; Shin, Y.C.; Cho, S.G.; Ko, S.G. SH003 Represses Tumor Angiogenesis by Blocking VEGF Binding to VEGFR2. Oncotarget 2016, 7, 32969–32979. [Google Scholar] [CrossRef]
- Mouhid, L.; de Cedrón, M.G.; García-Carrascosa, E.; Reglero, G.; Fornari, T.; de Molina, A.R. Yarrow Supercritical Extract Exerts Antitumoral Properties by Targeting Lipid Metabolism in Pancreatic Cancer. PLoS ONE 2019, 14, e0214294. [Google Scholar] [CrossRef] [Green Version]
- Akasaka, H.; Sasaki, R.; Yoshida, K.; Takayama, I.; Yamaguchi, T.; Yoshida, H.; Mizushina, Y. Monogalactosyl Diacylglycerol, a Replicative DNA Polymerase Inhibitor, from Spinach Enhances the Anti-Cell Proliferation Effect of Gemcitabine in Human Pancreatic Cancer Cells. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 2517–2525. [Google Scholar] [CrossRef] [Green Version]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Koffi, E.K.; Sea, T.L.; Dodehe, Y.; Soro, S.Y. Effect of Solvent Type on Extraction of Polyphenols from Twenty Three Ivorian Plants. J. Anim. Plant Sci. 2010, 5, 550–558. [Google Scholar]
- Onyebuchi, C.; Kavaz, D. Effect of Extraction Temperature and Solvent Type on the Bioactive Potential of Ocimum gratissimum L. Extracts. Sci. Rep. 2020, 10, 21760. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The Role of Polyphenols in Overcoming Cancer Drug Resistance: A Comprehensive Review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Shah, U.; Shah, R.; Acharya, S.; Acharya, N. Novel Anticancer Agents from Plant Sources. Chin. J. Nat. Med. 2013, 11, 16–23. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Pant, A.B.; Shukla, Y.; Chaudhari, B.; Kumar, P.; Gupta, K.C. Bromelain Nanoparticles Protect against 7,12-Dimethylbenz[a]Anthracene Induced Skin Carcinogenesis in Mouse Model. Eur. J. Pharm. Biopharm. 2015, 91, 35–46. [Google Scholar] [CrossRef]
- Mesas, C.; Garcés, V.; Martínez, R.; Ortiz, R.; Doello, K.; Dominguez-Vera, J.M.; Bermúdez, F.; Porres, J.M.; López-Jurado, M.; Melguizo, C.; et al. Colon Cancer Therapy with Calcium Phosphate Nanoparticles Loading Bioactive Compounds from Euphorbia Lathyris: In Vitro and in Vivo Assay. Biomed. Pharmacother. 2022, 155, 113723. [Google Scholar] [CrossRef]
- Al-Harbi, L.N.; Al-Shammari, G.M.; Subash-Babu, P.; Mohammed, M.A.; Alkreadees, R.A.; Yagoub, A.E.A. Cinchona Officinalis Phytochemicals-Loaded Iron Oxide Nanoparticles Induce Cytotoxicity and Stimulate Apoptosis in MCF-7 Human Breast Cancer Cells. Nanomaterials 2022, 12, 3393. [Google Scholar] [CrossRef]
- Venkatas, J.; Daniels, A.; Singh, M. The Potential of Curcumin-Capped Nanoparticle Synthesis in Cancer Therapy: A Green Synthesis Approach. Nanomaterials 2022, 12, 3201. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Mahapatra, P.K.; das Saha, K. Morin Encapsulated Chitosan Nanoparticles (MCNPs) Ameliorate Arsenic Induced Liver Damage through Improvement of the Antioxidant System and Prevention of Apoptosis and Inflammation in Mice. Nanoscale Adv. 2022, 4, 2857–2872. [Google Scholar] [CrossRef]
- Bhatia, D.; Mandal, A.; Nevo, E.; Bishayee, A. Apoptosis-Inducing Effects of Extracts from Desert Plants in HepG2 Human Hepatocarcinoma Cells. Asian Pac. J. Trop. Biomed. 2015, 5, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Zahri, S.; Razavi, S.M.; Niri, F.H.; Mohammadi, S. Induction of Programmed Cell Death by Prangos Uloptera, a Medicinal Plant. Biol. Res. 2009, 42, 517–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majid, M.Z.; Mohamad Zaini, Z.; Abdul Razak, F. Apoptosis-Inducing Effect of Three Medicinal Plants on Oral Cancer Cells KB and ORL-48. Sci. World J. 2014, 2014, 125353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133. [Google Scholar] [CrossRef] [Green Version]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and Gemcitabine in Patients with Advanced Pancreatic Cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Crane, E.A.; Gademann, K. Capturing Biological Activity in Natural Product Fragments by Chemical Synthesis. Angew. Chem. Int. Ed. 2016, 55, 3882–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquemard, C.; Kellenberger, E. A Bright Future for Fragment-Based Drug Discovery: What Does It Hold? Expert Opin. Drug Discov. 2019, 14, 413–416. [Google Scholar] [CrossRef] [Green Version]
- Casertano, M.; Genovese, M.; Piazza, L.; Balestri, F.; del Corso, A.; Vito, A.; Paoli, P.; Santi, A.; Imperatore, C.; Menna, M. Identifying Human PTP1B Enzyme Inhibitors from Marine Natural Products: Perspectives for Developing of Novel Insulin-Mimetic Drugs. Pharmaceuticals 2022, 15, 325. [Google Scholar] [CrossRef]
- Sun, S.; Kim, M.J.; Dibwe, D.F.; Omar, A.M.; Athikomkulchai, S.; Phrutivorapongkul, A.; Okada, T.; Tsuge, K.; Toyooka, N.; Awale, S. Anti-Austerity Activity of Thai Medicinal Plants: Chemical Constituents and Anti-Pancreatic Cancer Activities of Kaempferia Parviflora. Plants 2021, 10, 229. [Google Scholar] [CrossRef]
- Chang, H.W.; Liu, P.F.; Tsai, W.L.; Hu, W.H.; Hu, Y.C.; Yang, H.C.; Lin, W.Y.; Weng, J.R.; Shu, C.W. Xanthium Strumarium Fruit Extract Inhibits ATG4B and Diminishes the Proliferation and Metastatic Characteristics of Colorectal Cancer Cells. Toxins 2019, 11, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Önder, A. Anticancer Activity of Natural Coumarins for Biological Targets. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 64, pp. 85–109. [Google Scholar]
- Wardecki, T.; Brötz, E.; de Ford, C.; von Loewenich, F.D.; Rebets, Y.; Tokovenko, B.; Luzhetskyy, A.; Merfort, I. Endophytic Streptomyces in the Traditional Medicinal Plant Arnica montana L.: Secondary Metabolites and Biological Activity. Antonie Van Leeuwenhoek 2015, 108, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Momose, I.; Adachi, H.; Yamazaki, Y.; Sawa, R.; Ohba, S.I.; Kawada, M. Human Pancreatic Cancer Cells under Nutrient Deprivation Are Vulnerable to Redox System Inhibition. J. Biol. Chem. 2020, 295, 16678–16690. [Google Scholar] [CrossRef] [PubMed]


| Family | Species | N° Article | Part of Plant | Mechanism of Action |
|---|---|---|---|---|
| Araliaceae | Oplopanax horridus (Sm.) Miq. | 3 | Stem; Root | Apoptosis |
| Myrtaceae | Eugenia involucrata DC. | 1 | Fruits; Seed | Apoptosis |
| Cleistocalyx operculatus (Roxb.) | 1 | Bud | ||
| Eucalyptus robusta Sm., Eucalyptus microcorys F.Muell. and Eucalyptus saligna Sm. | 2 | Leaves | ||
| Syzygium aromaticum L. | 1 | Entire plant | KRAS mutation | |
| Acanthaceae | Clinacanthus nutans (Burm.f.) Lindau | 1 | Leaves Stem | Apoptosis |
| Rosaceae | Prunus armeniaca L. | 1 | Seed | Apoptosis |
| Campanulaceae | Codonopsis cordifolioidea P.C. Tsoong | 1 | Root | Apoptosis |
| Amaranthaceae | Spinacia oleracea L. | 2 | Leaves | Apoptosis |
| Rutaceae | Citrus hystrix DC. | 1 | Fruit | KRAS mutation |
| Annonaceae | Uvaria dac | 1 | Stem | KRAS mutation |
| Colchicaceae | Gloriosa superba L. | 1 | Seed | Apoptosis |
| Lamiaceae | Scutellaria baicalensis Georgi | 1 | Root | Apoptosis |
| Ocimum sanctum L. | 1 | Leaves | Arrest cell cycle | |
| Asteraceae | Taraxacum officinale L. | 1 | Root | KRAS mutation |
| Vernonia anthelmintica L. | 1 | Fruit | ||
| Inula helenium L. | 1 | Rhizome Root | ||
| Malvaceae | Helicteres hirsuta Lour. | 1 | Stem | Arrest cell cycle |
| Pterospermum acerifolium L. | 1 | Bark | ||
| Moringaceae | Moringa oleífera Lam. | 1 | Leaves | Arrest cell cycle |
| Ranunculaceae | Pulsatilla koreana (Yabe ex Nakai) | 1 | Root | Other alterations |
| Fabaceae, Apiáceas, Cucrbitaceae | Astragalus membranaceus (Fisch.), Angelica gigas Nakai, Trichosanthes Kirilowii Maxim. | 1 | Entire plant | Other alterations |
| Meliaceae, Lauraceae | Meliae, Cinnamon, Sparganium | 1 | Fruit; Bark; Rhizome | Arrest cell cycle |
| Asteraceae | Achillea millefolium L. | 1 | Entire plant | Other alterations |
| Material (Reference) | Extraction Method | Isolated Compounds | Cell Line | IC50 | Mechanism of Action |
|---|---|---|---|---|---|
| Stem of Oplopanax Horridus (Sm.) Miq. [26] | Ethanol 70% for DCEE Ddw for DCWE | DCEE DCWE DCA (1–4) | PANC-1 BxPC-3 | DCEE: PANC-1: 5.5–5.8 µg/mL BxPC-3: 21–34 µg/mL DCWE: PANC-1: 4950 µg/mL DCA: PANC-1: 0.22–1.593 µg/mL BxPC-3: 0.82–1.404 µg/ml | DCEE and DCWE induced apoptosis and nuclear necrosis. DCA induced apoptosis by both the intrinsic and extrinsic pathways. |
| Dried root of Oplopanax horridus (Sm.) Miq. [27] | Ethanol 70% | DC DCA | PANC-1 BxPC-3 | DC: PANC-1: 0.0058% (v/v) BxPC-3: 0.021% (v/v) DCA: PANC-1: 0.73 µM BxPC-3: 2.71 µM | DC caused cell cycle arrest and apoptosis induction, increasing caspase-3 expression. DC and DCA decreased expression of BCL-2 and BAX mRNA. |
| Fruits and seeds of Eugenia involucrata DC. [28] | Ethanol 99.4% | FE SE | PANC-1 | SE: PANC-1: 645 µg/mL | SE caused apoptosis induction and increased ROS generation. |
| Bud of Cleistocalyx operculatus (Roxb.) [29] | Ethanol 70% | DMC | PANC-1 MIA PaCa-2 | DMC: PANC-1: 10.5 µM MIA PaCa-2: 12.2 µM | DMC produced apoptosis by increasing the activity of caspase-3 and -9 and inhibiting the expression of antiapoptotic protein such as BCL-2. |
| Fresh leaves of Eucalyptus robusta Sm., E. microcorys F.Muell and E. saligna Sm. [30] | Ethanol 70% Ddw | Ethanol and Ddw extract of both | MIA PaCa-2 BxPC-3 CFPAC-1 HPDE | Ethanol extract and ddw extract of E. microcorys: MIA PaCa-2: 64.66–86.05 µg/mL Ethanol extract of E. saligna: MIA PaCa-2: 115.52 µg/mL | Ddw extract of E.microcorys induce apoptosis through caspase-3/7 expression |
| Dried sedes of Gloriosa superba L. [31] | Ethanol 80% | GS GS2B | PANC-1 Panc02 | GS: PANC-1: 0.45–0.59 µg/mL Panc02: 0.17–0.19 µg/mL GS2B: Panc02: 9.49 µg/mL | In a Panc02 in vivo model, both extract increased caspase-3 levels in tumor cells and decreased ki67 expression |
| Leaves and stems of Clinacanthus nutans (Burm.f.) [33] | Methanol and dichloromethane for polar compounds Hexane and diethyl ether for nonpolars | LP LN SP SN | AsPC-1 BxPC-3 SW1990 | SN: AsPC-1: 31.21 µg/mL BxPC-3: 39.12 µg/mL SW1990: 30.91 µg/ml | Synergistic action with GMZ, producing an increase in proapoptotic proteins such as BAX and a decrease in antiapoptotic proteins such as BCL-2, XIAP and CIAP-2. |
| Roots of Codonopsis cordifolioidea P.C. Tsoong [34] | Methanol 70% | Cordifoliketones A | BxPC-3 PANC-1 AsPC-1 | Cordifoliketones A: AsPC-1: 5.56 µg/mL BxPC-3: 4.26 µg/mL PANC-1: 4.18 µg/mL | Apoptosis induction by increased expression of proapoptotic proteins (BAX, BAD) and decreased expression of antiapoptotic proteins (BCL-2, BCL-XL). Cell migration inhibition and decreased in vivo tumor size. |
| Seeds of Prunus armeniaca L. [32] | Ethanol | BAEE | PANC-1 | BAEE: PANC-1: 704 µg/mL | Apoptosis induction by increased BAX and caspase-3 expression and BCL-2 inhibition. |
| Dried leaves of Spinacia oleracea L. [35] | Ethanol 70% | MGDG | MIA PaCa-2 PANC-1 AsPC-1 BxPC-3 | MGDG: MIA PaCa-2: 18.5 µM PANC-1: 25.6 µM AsPC-1: 22.7 µM BxPC-3: 26.9 µM | Apoptosis induction observed in MIA PaCa-2 cell line by increased cytochrome C levels in the cytosol, increased expression of PARP, caspase-3 and BAX and decreased expression of BCL-2 (antiapoptotic protein). Potentiation of the suppressive effects of radiation, both in MIA PaCa-2 in vitro and in vivo model. |
| Dried root of Scutellaria baicalensis Georgi [36] | Ethyl acetate | TFAE | BxPC-3 PANC-1 HPDE6c7 | TFAE: BxPC-3: 41.7 µg/mL (24 h) 12.3 µg/mL (48 h) 6.5 µg/mL (72 h) PANC-1: 47.4 µg/mL (24 h) 20.5 µg/mL (48 h) 8.9 µg/mL (72 h) | Induction of apoptosis by caspase-3/8, PARP and BID in BxPC-3, without action on BCL-2. Induction of autophagy, visible in increased LC3 II, through decreased activity in the PI3K/AKT/mTOR pathway. Tumor growth decreased and absence of healthy cells toxicity. |
| Material (Reference) | Extraction Method | Isolated Compounds | Cell Line | IC50 | Mechanism of Action |
|---|---|---|---|---|---|
| Root of Taraxacum officinale L. [37] | Ddw | DRE | BxPC-3 PANC-1 | DRE: 5 mg/mL in both cell lines. | Induction of autophagy. Apoptosis induction through destabilization of the mitochondrial membrane, causing the release of proapoptotic factors and increased caspase-8 activity. |
| Stem of Uvaria dac [38] | CH2Cl2 | GF | PANC-1 | GF: PANC-1: 14.5 µM | Increased cytotoxicity in PANC-1 cells grown in nutrient-free medium and AKT/mTOR inhibition. |
| Dried fruits of Vernonia anthelmintica L. [39] | Ethanol 80% | -Eriodictyol -Apigenin -Butein -Butin -Isorhamnetin -Sulfuretin -Luteolin -3,5-O-DCAME -3,4-O-DCAME | PANC-1 | Isorhamnetin: PANC-1: 19.6 µM Luteolin: PANC-1: 18.1 µM | Isorhamnetin produced S-phase arrest of tumor cells, inhibition of the RAS/MAPK pathway through inhibition of MEK phosphorylation and suppression of in vitro cell migration. |
| Fruits of Citrus hystrix DC. [40] | Ethanol 70% | -(R)-(+)-OM -(R)-(+)-OHI -(S)-(−)-O -(R)-(+)-P -Bergamottin -(R)-(+)-6HMBD -7-hydroxycoumarin | PANC-1 MIA PaCa-2 PSN-1 | Bergamottin: PANC-1: 4.6 µM MIA PaCa-2: 2.2 µM PSN-1: 9.4 µM | Bergamottin produced selective cytotoxicity on cells in a poor-nutrient medium, inhibited AKT expression and cell migration |
| Rhizome and dried roots of Inula helenium L. [41] | Ethanol 95% | EEIHL | CFPAC-1 | EEIHL: CFPAC-1: 4.3 µg/mL | Cell cycle arrest in G0/G1 phase, depolarization of membrane potential, apoptosis induction, inhibition of AKT and STAT-3 phosphorylation and cell migration inhibition by augmented expression of E-cadherin. |
| Material (Reference) | Extraction Method | Isolated Compounds | Cell Line | IC50 | Mechanism of Action |
|---|---|---|---|---|---|
| Stem of Helicteres hirsuta Lour. [42] | Methanol 40% | Extract enriched in saponin. 6 fractions were obtained from the extract (F0–F5) by HPLC. | MIA PaCa-2 BxPC-3 | F1: MiapaCa-2: 7.76 µg/mL BxPC-3: 17.12 µg/mL F2: MiapaCa-2: 4.54 µg/mL BxPC-3: 9.25 µg/mL F3: MiapaCa-2: 3.85 µg/mL BxPC-3: 3.71 µg/mL F4: MiapaCa-2: 3.88 µg/mL BxPc-3: 5.16 µg/mL F5: MiapaCa-2: 3.11 µg/mL BxPC-3: 4.23 µg/mL | Fractions F2, F3, F4, F5 produced cell cycle arrest in phase S. |
| Dried leaves of Ocimum sanctum L. (Holy Basil) [43] | Ethanol 100% | EEOL AEOL | AsPC-1 MIA PaCa-2 | EEOL: AsPC-1: 46 µg/mL MiapaCa-2: 69 µg/mL | Cell cycle arrest in G2/S in MIA PaCa-2 cells Decreased expression of NF-κB, increased expression of proapoptotic protein Bad. Decreased expression of BCL-2 and BCL-XL, in addition to increasing of BAD and E-cadherin in in vivo conditions. |
| Bark of Pterospermum acerifolium L. [44] | Ethanol 70% | PaEBE | PANC-1 | PaEBE: PANC-1: 74.22 µg/mL | Cell cycle arrest in G1 phase, increased ROS production and alteration of mitochondrial membrane, inducing apoptosis. |
| Combination of Meliae fructus, Cinnamon bark, Sparganium rhizome [45] | Ethanol 40% | H3 | PANC-1 | H3: PANC-1: 0.07 mg/mL | Cell cycle arrest in G0/G1 phase, cell migration inhibition, decreased expression in mRNA expression of genes associated with apoptosis (JAK2, CXCR4, XIAP) and increased cytochrome C levels in the cytoplasm. |
| Leaves of Eucalyptus microcorys F.Muell [47] | Ddw | Fractions (F1–F5) | MIA PaCa-2 BxPC-3 CFPAC-1 | F1: MIA PaCa-2: 93.11 µg/mL | Induced cell cycle arrest in G2/M phase, higher in combination with GMZ and apoptosis induction through decreased BCL-2 and increased BAX expression. |
| Dried root of Oplopanax horridus (Sm.) [46] | Ethanol 70% | DC DCA | PANC-1 | DC: 2D culture: 1/(17200) dilution 3D culture: 1/(3311) dilution DCA: 2D culture: 0.73 µM 3D culture: 3.15 µM | Cell cycle arrest observed in S phase in 3D cultured cells. |
| Leaves of Moringa oleífera Lam. [48] | Ddw | Aqueous extract | PANC-1 COLO 357 p34 | PANC-1: 1.1 mg/mL COLO 357: 1.8 mg/mL p34: 1.5 mg/mL | Increased number of PANC-1 cells in sub-G1 phase, decreased expression of proteins of the NF-kB signaling pathway (p65, IkBa) and possible synergistic action in combination with cisplatin. |
| Material (Reference) | Extraction Method | Isolated Compounds | Cell Line | IC50 | Mechanism of Action |
|---|---|---|---|---|---|
| Root of Pulsatilla koreana (Yabe ex Nakai) [49] | Ethanol 50% | SB365 | PANC-1 MIA PaCa-2 BxPC-3 AsPC-1 | SB365: 0.8–2 µM in all cell lines | Inhibition of the expression of HIF-1α and VEGF in a hypoxic environment with antiangiogenic effects also in mice, increased cytosolic cytochrome C and caspase-3 and decreased BCL-2 levels. Decreased number of KI67-positive cells. |
| Astragalus membranaceus, Angelica gigas and Trichosanthes Kirilowii Maximowicz [50] | Ethanol 30% | SH003 | HUVECs | SH003: 0.23–2.67 µg/mL | Inhibits VEGF/VEGFR-2-mediated angiogenesis in vitro, retarding the growth of Panc-28-LUC cells in mice. Reduction of KI67, p-VEGFR2 and MMP-9 levels and increased levels of caspase-3. |
| Achillea millefolium (Yarrow) [51] | CO2 | Yarrow SFE | MIA PaCa-2 PANC-1 | Yarrow SFE: MIA PaCa-2: 31.45 µg/mL | Decreased expression of SREBF1, FASN and SCD, involved in the production of fatty acids (overexpressed in PANC-1 and MIA PaCa-2 cells). Inhibition of in vivo tumor growth. |
| Spinacia oleracea L. [52] | Ethanol 70% | MGDG | PANC-1 BxPC-3 MIA PaCa-2 | MGDG: PANC-1: 22 nM BxPC-3: 15.1 nM MIA PaCa-2: 18.8 nM | Selective inhibition of α, δ and ε polymerases (with IC50 of 10.7–22 µM) and gamma, with IC50 of 35.1 µM. Induction of apoptosis on MIA PaCa-2. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesas, C.; Quiñonero, F.; Doello, K.; Revueltas, J.L.; Perazzoli, G.; Cabeza, L.; Prados, J.; Melguizo, C. Active Biomolecules from Vegetable Extracts with Antitumoral Activity against Pancreas Cancer: A Systematic Review (2011–2021). Life 2022, 12, 1765. https://doi.org/10.3390/life12111765
Mesas C, Quiñonero F, Doello K, Revueltas JL, Perazzoli G, Cabeza L, Prados J, Melguizo C. Active Biomolecules from Vegetable Extracts with Antitumoral Activity against Pancreas Cancer: A Systematic Review (2011–2021). Life. 2022; 12(11):1765. https://doi.org/10.3390/life12111765
Chicago/Turabian StyleMesas, Cristina, Francisco Quiñonero, Kevin Doello, José L. Revueltas, Gloria Perazzoli, Laura Cabeza, Jose Prados, and Consolación Melguizo. 2022. "Active Biomolecules from Vegetable Extracts with Antitumoral Activity against Pancreas Cancer: A Systematic Review (2011–2021)" Life 12, no. 11: 1765. https://doi.org/10.3390/life12111765
APA StyleMesas, C., Quiñonero, F., Doello, K., Revueltas, J. L., Perazzoli, G., Cabeza, L., Prados, J., & Melguizo, C. (2022). Active Biomolecules from Vegetable Extracts with Antitumoral Activity against Pancreas Cancer: A Systematic Review (2011–2021). Life, 12(11), 1765. https://doi.org/10.3390/life12111765