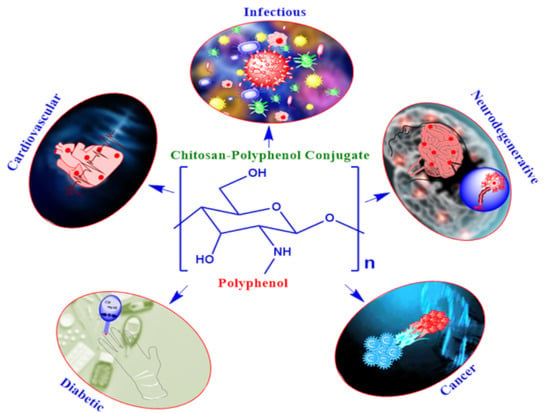
Chitosan-Polyphenol Conjugates for Human Health
Abstract
:1. Introduction
2. Chitosan-Polyphenol Conjugates
2.1. Chitosan-Gallic Acid Conjugates
2.2. Chitosan-Curcumin Conjugates
2.3. Chitosan-Catechin Conjugates
2.4. Chitosan-Quercetin Conjugates
3. Conclusions and Future Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shutava, T.G.; Balkundi, S.S.; Vangala, P.; Steffan, J.J.; Bigelow, R.L.; Cardelli, J.A.; O’Neal, D.P.; Lvov, Y.M. Layer-by-Layer-Coated Gelatin Nanoparticles as a Vehicle for Delivery of Natural Polyphenols. ACS Nano 2009, 3, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.J.; Moore, J.; Song, J.; Tsiani, E.L. Inhibition of Non-Small Cell Lung Cancer Proliferation and Survival by Rosemary Extract Is Associated with Activation of ERK and AMPK. Life 2021, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [Green Version]
- Higbee, J.; Solverson, P.; Zhu, M.; Carbonero, F. The Emerging Role of Dark Berry Polyphenols in Human Health and Nutrition. Food Front. 2022, 3, 3–27. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Chen, H.; Chen, K.; Tao, W.; Ouyang, X.; Mei, L.; Zeng, X. Polyphenol-Based Hydrogels: Pyramid Evolution from Crosslinked Structures to Biomedical Applications and the Reverse Design. Bioact. Mater. 2022, 17, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Yang, P.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Polyphenol Scaffolds in Tissue Engineering. Mater. Horiz. 2021, 8, 145–167. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Saeedi, P.; Izadifar, Z.; Bekhit, A.A.; Khademhosseini, A. Polyphenol Uses in Biomaterials Engineering. Biomaterials 2018, 167, 91–106. [Google Scholar] [CrossRef]
- Kawakami, S.; Morinaga, M.; Tsukamoto-Sen, S.; Mori, S.; Matsui, Y.; Kawama, T. Constituent Characteristics and Functional Properties of Passion Fruit Seed Extract. Life 2021, 12, 38. [Google Scholar] [CrossRef]
- Meccariello, R.; D’Angelo, S. Impact of Polyphenolic-Food on Longevity: An Elixir of Life. An Overview. Antioxidants 2021, 10, 507. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.-H. From Fighting Critters to Saving Lives: Polyphenols in Plant Defense and Human Health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Chowdhary, V.; Alooparampil, S.; Pandya, R.V.; Tank, J.G. Physiological Function of Phenolic Compounds in Plant Defense System. In Phenolic Compounds-Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology-Perspectives in Agriculture; IntechOpen: London, UK, 2022. [Google Scholar]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; pp. 517–532. [Google Scholar]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of Phenols and Polyphenols in Plant Defense Response to Biotic and Abiotic Stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. [Google Scholar]
- Singh, S.; Kaur, I.; Kariyat, R. The Multifunctional Roles of Polyphenols in Plant-Herbivore Interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Biondo, E.; Kolchinski, E.M.; da Silva, L.F.S.; Corrêa, A.P.F.; Bach, E.; Brandelli, A. Total Polyphenols, Antioxidant, Antimicrobial and Allelopathic Activities of Spend Coffee Ground Aqueous Extract. Waste Biomass Valorization 2017, 8, 439–442. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, Antioxidant and Antimicrobial Activities of Leaf and Bark Extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Trošt, K.; Klančnik, A.; Mozetič Vodopivec, B.; Sternad Lemut, M.; Jug Novšak, K.; Raspor, P.; Smole Možina, S. Polyphenol, Antioxidant and Antimicrobial Potential of Six Different White and Red Wine Grape Processing Leftovers. J. Sci. Food Agric. 2016, 96, 4809–4820. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Li, X.; Gao, P.; Tan, J.; Xiong, K.; Maitz, M.F.; Pan, C.; Wu, H.; Chen, Y.; Yang, Z.; Huang, N. Assembly of Metal–Phenolic/Catecholamine Networks for Synergistically Anti-Inflammatory, Antimicrobial, and Anticoagulant Coatings. ACS Appl. Mater. Interfaces 2018, 10, 40844–40853. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, X.; Gao, M.; Liao, X.; Shi, B. Polyphenol-Grafted Collagen Fiber as Reductant and Stabilizer for One-Step Synthesis of Size-Controlled Gold Nanoparticles and Their Catalytic Application to 4-Nitrophenol Reduction. Green Chem. 2011, 13, 651. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, X.; Liang, X.; Liang, J.; Zhang, C.; Yang, J.; Wang, C.; Kong, D.; Sun, H. ROS-Responsive Capsules Engineered from Green Tea Polyphenol–Metal Networks for Anticancer Drug Delivery. J. Mater. Chem. B 2018, 6, 1000–1010. [Google Scholar] [CrossRef]
- Khanam, A.; Ahmad, A.; Iftikhar, N.; Ali, Q.; Fatima, T.; Alswailmi, F.K.; Hussain, A.I.; Alnasser, S.M.A.; Akhtar, J. Variation in Phenolic Profile, Antioxidant, and Anti-Inflammatory Activities of Salvadora oleoides Decene. and Salvadora persica L. Fruits and Aerial Part Extracts. Life 2022, 12, 1446. [Google Scholar] [CrossRef]
- Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Jawhari, F.Z.; Al Kamaly, O.M.; Imtara, H.; Grafov, A.; Bari, A.; Bousta, D. An Insight into the Anxiolytic and Antidepressant-Like Proprieties of Carum carvi L. and Their Association with Its Antioxidant Activity. Life 2021, 11, 207. [Google Scholar] [CrossRef]
- Sirše, M. Effect of Dietary Polyphenols on Osteoarthritis—Molecular Mechanisms. Life 2022, 12, 436. [Google Scholar] [CrossRef]
- Shaik, R.A.; Eid, B.G. Piceatannol Affects Gastric Ulcers Induced by Indomethacin: Association of Antioxidant, Anti-Inflammatory, and Angiogenesis Mechanisms in Rats. Life 2022, 12, 356. [Google Scholar] [CrossRef]
- Ko, Y.H.; Jeong, M.; Jang, D.S.; Choi, J.-H. Gomisin L1, a Lignan Isolated from Schisandra Berries, Induces Apoptosis by Regulating NADPH Oxidase in Human Ovarian Cancer Cells. Life 2021, 11, 858. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. In Vivo Formed Metabolites of Polyphenols and Their Biological Efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef]
- Pastene, E.; Speisky, H.; García, A.; Moreno, J.; Troncoso, M.; Figueroa, G. In Vitro and in Vivo Effects of Apple Peel Polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2010, 58, 7172–7179. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.; Abia, R.; Saura-Calixto, F. Polyphenols as Dietary Fiber Associated Compounds. Comparative Study on in Vivo and in Vitro Properties. J. Agric. Food Chem. 1994, 42, 1481–1487. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Smith, C. Are Polyphenol Antioxidants at the Root of Medicinal Plant Anti-Cancer Success? J. Ethnopharmacol. 2019, 229, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P.; et al. Recent Advances in Research on Polyphenols: Effects on Microbiota, Metabolism, and Health. Mol. Nutr. Food Res. 2022, 66, 2100670. [Google Scholar] [CrossRef]
- Dolati, S.; Babaloo, Z.; Ayromlou, H.; Ahmadi, M.; Rikhtegar, R.; Rostamzadeh, D.; Roshangar, L.; Nouri, M.; Mehdizadeh, A.; Younesi, V.; et al. Nanocurcumin Improves Regulatory T-Cell Frequency and Function in Patients with Multiple Sclerosis. J. Neuroimmunol. 2019, 327, 15–21. [Google Scholar] [CrossRef]
- Yang, J.; Song, S.; Li, J.; Liang, T. Neuroprotective Effect of Curcumin on Hippocampal Injury in 6-OHDA-Induced Parkinson’s Disease Rat. Pathol.-Res. Pract. 2014, 210, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Ahmadi, M.; Aghebti-Maleki, L.; Nikmaram, A.; Marofi, F.; Rikhtegar, R.; Ayromlou, H.; Yousefi, M. Nanocurcumin Is a Potential Novel Therapy for Multiple Sclerosis by Influencing Inflammatory Mediators. Pharmacol. Rep. 2018, 70, 1158–1167. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bahar, E.; Kim, J.-Y.; Yoon, H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, INOS/NF-ΚB and HO-1/Nrf2 Pathways. Int. J. Mol. Sci. 2017, 18, 1989. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential Neuroprotective Properties of Epigallocatechin-3-Gallate (EGCG). Nutr. J. 2015, 15, 60. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Rogers, J.; Xie, J. Brain Iron Metabolism Dysfunction in Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 3078–3101. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Xu, Y. Resveratrol Protects Vascular Smooth Muscle Cells against High Glucose-Induced Oxidative Stress and Cell Proliferation in Vitro. Med. Sci. Monit. Basic Res. 2014, 20, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liu, Q.; Lin, J.; Wang, Y.; Chen, S.; Hou, J. GW27-E0657 Resveratrol Protects against Oxidized LDL-Induced Foam Cells Formation and Apoptosis through Inhibition of ER Stress and Downregulation of CD36. J. Am. Coll. Cardiol. 2016, 68, C26. [Google Scholar] [CrossRef]
- Xiao, J.B.; Hogger, P. Dietary Polyphenols and Type 2 Diabetes: Current Insights and Future Perspectives. Curr. Med. Chem. 2014, 22, 23–38. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic Profiles of 20 Canadian Lentil Cultivars and Their Contribution to Antioxidant Activity and Inhibitory Effects on α-Glucosidase and Pancreatic Lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel Insights of Dietary Polyphenols and Obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ma, X.; An, Y.; Sun, Y.; Dou, W.; Li, M.; Bao, H.; Zhang, C. Green Tea Polyphenols Alleviate Hydrogen Peroxide-Induced Oxidative Stress, Inflammation, and Apoptosis in Bovine Mammary Epithelial Cells by Activating ERK1/2–NFE2L2–HMOX1 Pathways. Front. Vet. Sci. 2022, 8, 804241. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, Y.; Yu, Y.; Zeng, Y. Enhanced Antibacterial Activity of Curcumin by Combination with Metal Ions. Colloid Interface Sci. Commun. 2018, 25, 1–6. [Google Scholar] [CrossRef]
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Eran Nagar, E.; Okun, Z.; Shpigelman, A. Digestive Fate of Polyphenols: Updated View of the Influence of Chemical Structure and the Presence of Cell Wall Material. Curr. Opin. Food Sci. 2020, 31, 38–46. [Google Scholar] [CrossRef]
- Ray, S.K.; Mukherjee, S. Evolving Interplay Between Dietary Polyphenols and Gut Microbiota—An Emerging Importance in Healthcare. Front. Nutr. 2021, 8, 634944. [Google Scholar] [CrossRef]
- Cirillo, G.; Kraemer, K.; Fuessel, S.; Puoci, F.; Curcio, M.; Spizzirri, U.G.; Altimari, I.; Iemma, F. Biological Activity of a Gallic Acid−Gelatin Conjugate. Biomacromolecules 2010, 11, 3309–3315. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Kim, S.-K.; Ahn, C.-B.; Je, J.-Y. Preparation, Characterization, and Antioxidant Properties of Gallic Acid-Grafted-Chitosans. Carbohydr. Polym. 2011, 83, 1617–1622. [Google Scholar] [CrossRef]
- Kim, B.; Kang, B.; Vales, T.P.; Yang, S.K.; Lee, J.; Kim, H.-J. Polyphenol-Functionalized Hydrogels Using an Interpenetrating Chitosan Network and Investigation of Their Antioxidant Activity. Macromol. Res. 2018, 26, 35–39. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and Antiradical SiO2 Nanoparticles Covalently Functionalized with Gallic Acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Socaci, S.; Farcas, A.; Socaciu, C.; Danciu, C.; Stanila, A.; Diaconeasa, Z. Novel Delivery Systems of Polyphenols and Their Potential Health Benefits. Pharmaceuticals 2021, 14, 946. [Google Scholar] [CrossRef]
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Farina, G.; Iemma, F.; Picci, N. Polyphenols and Their Formulations. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 29–45. [Google Scholar]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of Synergistic Polyphenol Combinations Using Biopolymer-Based Systems: Advances in Physicochemical Properties, Stability and Bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar] [CrossRef]
- Ohara, M.; Ohyama, Y. Delivery and Application of Dietary Polyphenols to Target Organs, Tissues and Intracellular Organelles. Curr. Drug Metab. 2014, 15, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, Q.; Wu, F.; Dai, Y.; Chen, X. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, 2007356. [Google Scholar] [CrossRef]
- Vittorio, O.; Curcio, M.; Cojoc, M.; Goya, G.F.; Hampel, S.; Iemma, F.; Dubrovska, A.; Cirillo, G. Polyphenols Delivery by Polymeric Materials: Challenges in Cancer Treatment. Drug Deliv. 2017, 24, 162–180. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Fan, Y.; Yan, J.; Yang, M. Engineering Polyphenol-Based Polymeric Nanoparticles for Drug Delivery and Bioimaging. Chem. Eng. J. 2022, 439, 135661. [Google Scholar] [CrossRef]
- Pan, Y.; Xia, Q.; Xiao, H. Cationic Polymers with Tailored Structures for Rendering Polysaccharide-Based Materials Antimicrobial: An Overview. Polymers (Basel) 2019, 11, 1283. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial Cationic Polymers: From Structural Design to Functional Control. Polym. J. 2018, 50, 33–44. [Google Scholar] [CrossRef]
- Hwang, S.J.; Davis, M.E. Cationic Polymers for Gene Delivery: Designs for Overcoming Barriers to Systemic Administration. Curr. Opin. Mol. Ther. 2001, 3, 183–191. [Google Scholar] [PubMed]
- Chen, C.-K.; Huang, P.-K.; Law, W.-C.; Chu, C.-H.; Chen, N.-T.; Lo, L.-W. Biodegradable Polymers for Gene-Delivery Applications. Int. J. Nanomed. 2020, 15, 2131–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, F.; Leer, K.; Martin, L.; Mapfumo, P.; Solomun, J.I.; Kuchenbrod, M.T.; Hoeppener, S.; Brendel, J.C.; Traeger, A. The Impact of Anionic Polymers on Gene Delivery: How Composition and Assembly Help Evading the Toxicity-Efficiency Dilemma. J. Nanobiotechnol. 2021, 19, 292. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, H.; Luo, Y. Chitosan-Based Oral Colon-Specific Delivery Systems for Polyphenols: Recent Advances and Emerging Trends. J. Mater. Chem. B 2022, 10, 7328–7348. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, S.-J.; Oh, M.-K.; Khan, A. Antibacterial Properties of Main-Chain Cationic Polymers Prepared through Amine–Epoxy ‘Click’ Polymerization. RSC Adv. 2020, 10, 26752–26755. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.; de Melo Carrasco, L. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [Green Version]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Cotrim Marques, S.; Marques Nunes, P.; Pedrosa, R.; Leandro, S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [Green Version]
- Park, P.-J.; Je, J.-Y.; Kim, S.-K. Free Radical Scavenging Activities of Differently Deacetylated Chitosans Using an ESR Spectrometer. Carbohydr. Polym. 2004, 55, 17–22. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Chelation Capability of Chitosan and Chitosan Derivatives: Recent Developments in Sustainable Corrosion Inhibition and Metal Decontamination Applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100184. [Google Scholar] [CrossRef]
- Pasanphan, W.; Chirachanchai, S. Conjugation of Gallic Acid onto Chitosan: An Approach for Green and Water-Based Antioxidant. Carbohydr. Polym. 2008, 72, 169–177. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Polyphenol-Chitosan Conjugates: Synthesis, Characterization, and Applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, F.; Fang, Y.; Yang, W.; An, X.; Zhao, L.; Xin, Z.; Cao, L.; Hu, Q. Cytotoxicity and Apoptotic Effects of Tea Polyphenol-Loaded Chitosan Nanoparticles on Human Hepatoma HepG2 Cells. Mater. Sci. Eng. C 2014, 36, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Riccucci, G.; Ferraris, S.; Reggio, C.; Bosso, A.; Örlygsson, G.; Ng, C.H.; Spriano, S. Polyphenols from Grape Pomace: Functionalization of Chitosan-Coated Hydroxyapatite for Modulated Swelling and Release of Polyphenols. Langmuir 2021, 37, 14793–14804. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Sun, X.; Raza, H.; Aslam Khan, M.; Ma, H.; Ren, X. Fabrication and Characterization of Quercetin Loaded Casein Phosphopeptides-Chitosan Composite Nanoparticles by Ultrasound Treatment: Factor Optimization, Formation Mechanism, Physicochemical Stability and Antioxidant Activity. Ultrason. Sonochem. 2021, 80, 105830. [Google Scholar] [CrossRef]
- Zhou, J.; Li, N.; Liu, P.; Liu, Z.; Gao, L.; Jiao, T. Preparation of Fluorescently Labeled Chitosan-Quercetin Drug-Loaded Nanoparticles with Excellent Antibacterial Properties. J. Funct. Biomater. 2022, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, H.J.; Chevallier, P.; Copes, F.; Simch, F.H.; da Silva Veloso, F.; Genevro, G.M.; Mantovani, D. Quercetin-Crosslinked Chitosan Films for Controlled Release of Antimicrobial Drugs. Front. Bioeng. Biotechnol. 2022, 10, 814162. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Kimura, T.; Kishida, A. Controlling Coupling Reaction of EDC and NHS for Preparation of Collagen Gels Using Ethanol/Water Co-Solvents. Macromol. Biosci. 2008, 8, 32–37. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P. Antimicrobial Chitosan Conjugates: Current Synthetic Strategies and Potential Applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Valli, E.; Farfalla, A.; Leggio, A.; Nicoletta, F.P.; Iemma, F. Chitosan–Quercetin Bioconjugate as Multi-Functional Component of Antioxidants and Dual-Responsive Hydrogel Networks. Macromol. Mater. Eng. 2019, 304, 1800728. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of Chitosan Micro/Nanoparticles for Pulmonary Drug Delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The Enzymatic Degradation and Swelling Properties of Chitosan Matrices with Different Degrees of N-Acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of Chitosan for Improvement of Quality and Shelf Life of Foods: A Review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef]
- Singh, A.; Mittal, A.; Benjakul, S. Chitosan, Chitooligosaccharides and Their Polyphenol Conjugates: Preparation, Bioactivities, Functionalities and Applications in Food Systems. Food Rev. Int. 2021, 37, 1–23. [Google Scholar] [CrossRef]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Yin, D.; Zeng, X. A Comparative Study on Chitosan/Gelatin Composite Films with Conjugated or Incorporated Gallic Acid. Carbohydr. Polym. 2017, 173, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, A.O.; Morimura, S.; Kida, K. Synthesis of Chitosan–Caffeic Acid Derivatives and Evaluation of Their Antioxidant Activities. J. Biosci. Bioeng. 2011, 111, 212–216. [Google Scholar] [CrossRef]
- Sakai, S.; Yamada, Y.; Zenke, T.; Kawakami, K. Novel Chitosan Derivative Soluble at Neutral PH and In-Situ Gellable via Peroxidase-Catalyzed Enzymatic Reaction. J. Mater. Chem. 2009, 19, 230–235. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Scher, J.; Muniglia, L. Enzymatic Synthesis of Chitosan Derivatives and Their Potential Applications. J. Mol. Catal. B Enzym. 2015, 112, 25–39. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic Acid: A Versatile Antioxidant with Promising Therapeutic and Industrial Applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Choubey, S.; Goyal, S.; Varughese, L.R.; Kumar, V.; Sharma, A.K.; Beniwal, V. Probing Gallic Acid for Its Broad Spectrum Applications. Mini-Rev. Med. Chem. 2018, 18, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial Activity of Gallic Acid against Food-Related Pseudomonas Strains and Its Use as Biocontrol Tool to Improve the Shelf Life of Fresh Black Truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Seeram, N.P.; Wyatt, H.; Henning, S.M.; Zhang, Y.; Ogden, L.G.; Dreher, M.; Hill, J.O. Safety and Antioxidant Activity of a Pomegranate Ellagitannin-Enriched Polyphenol Dietary Supplement in Overweight Individuals with Increased Waist Size. J. Agric. Food Chem. 2007, 55, 10050–10054. [Google Scholar] [CrossRef]
- Dludla, P.; Nkambule, B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosuru, R.Y.; Roy, A.; Das, S.K.; Bera, S. Gallic Acid and Gallates in Human Health and Disease: Do Mitochondria Hold the Key to Success? Mol. Nutr. Food Res. 2018, 62, 1700699. [Google Scholar] [CrossRef] [PubMed]
- AL Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent Developments of Gallic Acid Derivatives and Their Hybrids in Medicinal Chemistry: A Review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef] [PubMed]
- Lišková, J.; Douglas, T.E.L.; Beranová, J.; Skwarczyńska, A.; Božič, M.; Samal, S.K.; Modrzejewska, Z.; Gorgieva, S.; Kokol, V.; Bačáková, L. Chitosan Hydrogels Enriched with Polyphenols: Antibacterial Activity, Cell Adhesion and Growth and Mineralization. Carbohydr. Polym. 2015, 129, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.; Kan, J.; Jin, C. Synthesis of Chitosan-Gallic Acid Conjugate: Structure Characterization and in Vitro Anti-Diabetic Potential. Int. J. Biol. Macromol. 2013, 62, 321–329. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O.; Costa, L.S. Gallic Acid-Chitosan Conjugate Inhibits the Formation of Calcium Oxalate Crystals. Molecules 2019, 24, 2074. [Google Scholar] [CrossRef] [Green Version]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Tian, F.; Dahmani, F.Z.; Yang, H.; Yue, D.; He, S.; Zhou, J.; Yao, J. Curcumin-Carboxymethyl Chitosan (CNC) Conjugate and CNC/LHR Mixed Polymeric Micelles as New Approaches to Improve the Oral Absorption of P-Gp Substrate Drugs. Drug Deliv. 2016, 23, 3424–3435. [Google Scholar] [CrossRef] [Green Version]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of Curcumin in Alginate-Chitosan-Pluronic Composite Nanoparticles for Delivery to Cancer Cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhang, Y.; Han, S.; Chen, Y.; Li, B.; Liao, M.; Chen, W.; Deng, X.; Zhao, J.; Huang, B. Synthesis and in Vitro/in Vivo Anti-Cancer Evaluation of Curcumin-Loaded Chitosan/Poly(Butyl Cyanoacrylate) Nanoparticles. Int. J. Pharm. 2010, 400, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Mansour, H.M.; Zhang, Y.; Deng, X.; Chen, Y.; Wang, J.; Pan, Y.; Zhao, J. Reversion of Multidrug Resistance by Co-Encapsulation of Doxorubicin and Curcumin in Chitosan/Poly(Butyl Cyanoacrylate) Nanoparticles. Int. J. Pharm. 2012, 426, 193–201. [Google Scholar] [CrossRef]
- Popat, A.; Karmakar, S.; Jambhrunkar, S.; Xu, C.; Yu, C. Curcumin-Cyclodextrin Encapsulated Chitosan Nanoconjugates with Enhanced Solubility and Cell Cytotoxicity. Colloids Surf. B Biointerfaces 2014, 117, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, J.; Hong, Y. Development of Chitosan/Poly-γ-glutamic Acid/Pluronic/Curcumin Nanoparticles in Chitosan Dressings for Wound Regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Varaprasad, K.; Akbari-Fakhrabadi, A.; Hameed, A.S.H.; Sadiku, R. Biomolecule Chitosan, Curcumin and ZnO-Based Antibacterial Nanomaterial, via a One-Pot Process. Carbohydr. Polym. 2020, 249, 116825. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. Conjugation of Tea Catechins with Chitosan Nanoparticles. Food Hydrocoll. 2018, 84, 561–570. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z. Preparation and Characterization of Catechin-Grafted Chitosan with Antioxidant and Antidiabetic Potential. Int. J. Biol. Macromol. 2014, 70, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent Anti-Proliferative and pro-Apoptotic Effects of (−)-Epigallocatechin-3-Gallate Encapsulated in Chitosan Nanoparticles on Human Melanoma Cell Growth Both in Vitro and in Vivo. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Nihal, M.; Ahmad, N.; Mukhtar, H.; Wood, G.S. Anti-Proliferative and Proapoptotic Effects of (−)-Epigallocatechin-3-Gallate on Human Melanoma: Possible Implications for the Chemoprevention of Melanoma. Int. J. Cancer 2005, 114, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral Administration of Naturally Occurring Chitosan-Based Nanoformulated Green Tea Polyphenol EGCG Effectively Inhibits Prostate Cancer Cell Growth in a Xenograft Model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef]
- Anand David, A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Nan, W.; Ding, L.; Chen, H.; Khan, F.U.; Yu, L.; Sui, X.; Shi, X. Topical Use of Quercetin-Loaded Chitosan Nanoparticles Against Ultraviolet B Radiation. Front. Pharmacol. 2018, 9, 826. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. Amphiphilic Carboxymethyl Chitosan-Quercetin Conjugate with P-Gp Inhibitory Properties for Oral Delivery of Paclitaxel. Biomaterials 2014, 35, 7654–7665. [Google Scholar] [CrossRef]
- Vedakumari, W.S.; Ayaz, N.; Karthick, A.S.; Senthil, R.; Sastry, T.P. Quercetin Impregnated Chitosan–Fibrin Composite Scaffolds as Potential Wound Dressing Materials—Fabrication, Characterization and in Vivo Analysis. Eur. J. Pharm. Sci. 2017, 97, 106–112. [Google Scholar] [CrossRef]
- Patil, P.; Killedar, S. Formulation and Characterization of Gallic Acid and Quercetin Chitosan Nanoparticles for Sustained Release in Treating Colorectal Cancer. J. Drug Deliv. Sci. Technol. 2021, 63, 102523. [Google Scholar] [CrossRef]
- Mu, Y.; Wu, G.; Su, C.; Dong, Y.; Zhang, K.; Li, J.; Sun, X.; Li, Y.; Chen, X.; Feng, C. PH-Sensitive Amphiphilic Chitosan-Quercetin Conjugate for Intracellular Delivery of Doxorubicin Enhancement. Carbohydr. Polym. 2019, 223, 115072. [Google Scholar] [CrossRef]
- Rashedi, J.; Ghorbani Haghjo, A.; Mesgari Abbasi, M.; Dastranj Tabrizi, A.; Yaqoubi, S.; Sanajou, D.; Ashrafi Jigheh, Z.; Namvaran, A.; Mohammadi, A.; Mohammadi Khoshraj, J.; et al. Anti-Tumor Effect of Quercetin Loaded Chitosan Nanoparticles on Induced Colon Cancer in Wistar Rats. Adv. Pharm. Bull. 2019, 9, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Tzankova, V.; Aluani, D.; Kondeva-Burdina, M.; Yordanov, Y.; Odzhakov, F.; Apostolov, A.; Yoncheva, K. Hepatoprotective and Antioxidant Activity of Quercetin Loaded Chitosan/Alginate Particles in Vitro and in Vivo in a Model of Paracetamol-Induced Toxicity. Biomed. Pharmacother. 2017, 92, 569–579. [Google Scholar] [CrossRef] [PubMed]




















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattnaik, A.; Pati, S.; Samal, S.K. Chitosan-Polyphenol Conjugates for Human Health. Life 2022, 12, 1768. https://doi.org/10.3390/life12111768
Pattnaik A, Pati S, Samal SK. Chitosan-Polyphenol Conjugates for Human Health. Life. 2022; 12(11):1768. https://doi.org/10.3390/life12111768
Chicago/Turabian StylePattnaik, Ananya, Sanghamitra Pati, and Sangram Keshari Samal. 2022. "Chitosan-Polyphenol Conjugates for Human Health" Life 12, no. 11: 1768. https://doi.org/10.3390/life12111768
APA StylePattnaik, A., Pati, S., & Samal, S. K. (2022). Chitosan-Polyphenol Conjugates for Human Health. Life, 12(11), 1768. https://doi.org/10.3390/life12111768