The Anti-Genotoxic Activity of Wastewaters Produced after Water-Steam Distillation of Bulgarian Rosa damascena Mill. and Rosa alba L. Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
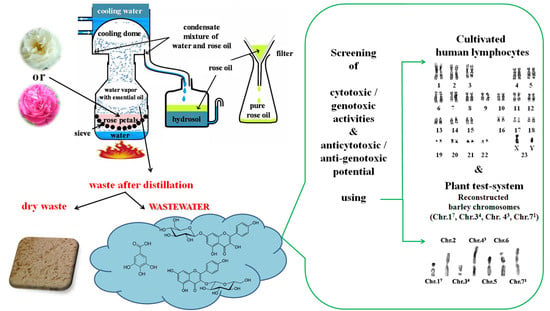
2.1. Preparation of Rose Wastewaters
2.2. Cytogenetic Analysis
2.2.1. Chemicals
2.2.2. Plant Test-System
2.2.3. Human Lymphocytes In Vitro
2.2.4. Endpoints
2.2.5. Statistics
3. Results
3.1. Cytotoxic and Genotoxic Effects of Wastewater from Distillation of Oils of R. alba and R. damascena
3.2. Anti-Cytotoxic and Anti-Genotoxic Activities of Wastewaters from Distillation of Oils of R. alba and R. damascena
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Widrlechner, M.P. History and utilization of Rosa damascena. Econ. Bot. 1981, 35, 42–58. [Google Scholar] [CrossRef]
- Schulz, H. Fragrance and pigments. In Odoriferous Substances and Pigments; Roberts, A.V., Debener, T., Gudin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 231–240. [Google Scholar]
- Özkan, G.; Sagdiç, O.; Göktürk-Baydar, N.; Baydar, H. Anti-oxidant and antibacterial activities of Rosa damascena flower extracts. Food Sci. Technol. Int. 2004, 10, 277–281. [Google Scholar] [CrossRef]
- Gochev, V.; Wlcek, K.; Buchbauer, G.; Stoyanova, A.; Dobreva, A.; Schmidt, E.; Jirovetz, L. Comparative Evaluation of Antimicrobial Activity and Composition of Rose Oils from Various Geographic Origins, in Particular Bulgarian Rose Oil. Nat. Prod. Commun. 2008, 3, 1063–1068. [Google Scholar] [CrossRef] [Green Version]
- Mahboubi, M. Rosa damascena as holy ancient herb with novel applications. J. Tradit. Complement. Med. 2016, 6, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gochev, V.; Jirovetz, L.; Wlcek, K.; Buchbauer, G.; Schmidt, E.; Stoyanova, A.; Dobreva, A. Chemical Composition and Antimicrobial Activity of Historical Rose Oil from Bulgaria. J. Essent. Oil Bear. Plants 2009, 12, 1–6. [Google Scholar] [CrossRef]
- Ulusoy, S.; Boşgelmez-Tınaz, G.; Seçilmiş-Canbay, H. Tocopherol, Carotene, Phenolic Contents and Antibacterial Properties of Rose Essential Oil, Hydrosol and Absolute. Curr. Microbiol. 2009, 59, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Kovacheva, N.; Rusanov, K.; Atanassov, I. Industrial cultivation of oil bearing rose and rose oil production in Bulgaria during 21st century, directions and challenges. Biotechnol. Biotechnol. Equip. 2010, 24, 1793–1798. [Google Scholar] [CrossRef] [Green Version]
- Koksal, N.; Aslancan, H.; Sadighazadi, S.; Kafkas, E. Chemical investigation on Rose damascena Mill. volatiles; effects of storage and drying conditions. Acta Sci. Pol. Hortorum Cultus 2015, 14, 105–114. [Google Scholar]
- Nunes, H.S.; Miguel, M.G. Rosa damascena essential oils: A brief review about chemical composition and biological properties. Trends Phytochem. Res. 2017, 1, 111–128. [Google Scholar]
- Antonova, D.V.; Medarska, Y.N.; Stoyanova, A.S.; Nenov, N.S.; Slavov, A.M.; Antonov, L.M. Chemical profile and sensory evaluation of Bulgarian rose (Rosa damascena Mill.) aroma products, isolated by different techniques. J. Essent. Oil Res. 2020, 33, 171–181. [Google Scholar] [CrossRef]
- Nedkov, N. Bulgarian Rose Oil of White Oil-Bearing Rose. Bulg. J. Agric. Sci. 2009, 15, 319–323. [Google Scholar]
- Gateva, S.; Jovtchev, G.; Chanev, C.; Georgieva, A.; Stankov, A.; Dobreva, A.; Mileva, M. Assessment of anti-cytotoxic, anti-genotoxic and antioxidant potential of Bulgarian Rosa alba L. essential oil. Caryologia Int. J. Cytol. Cytosyst. Cytogenet. 2020, 73, 71–88. [Google Scholar] [CrossRef]
- Schieber, A.; Mihalev, K.; Berardini, N.; Mollov, P.; Carle, R. Flavonol Glycosides from Distilled Petals of Rosa damascena Mill. Zeitschrift für Naturforschung C 2005, 60, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasova, T.; Kakalova, M.; Stefanof, L.; Petkova, M.; Stoyanova, A.; Damyanova, S.; Desyk, M. Chemical composition of essential oil from Rosa damascena Mill., growing in new region of Bulgaria. Ukr. Food J. 2016, 5, 492–498. [Google Scholar] [CrossRef]
- Erbas¸, S.; Baydar, H. Variation in scent compounds of oil bearing rose (Rosa damascena Mill.) produced by headspace solid phase microextraction, hydrodistillation and solvent extraction. Rec. Nat. Prod. 2016, 10, 555–565. [Google Scholar]
- Balev, D.; Vlahova, V.D.; Mihalev, K.; Shikov, V.; Dragoev, S.; Nikolov, V. Application of Natural Dietary Antioxidants in Broiler Feeds. J. Mt. Agric. Balk. 2015, 18, 224–232. [Google Scholar]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crop. Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Rusanov, K.; Garo, E.; Rusanova, M.; Fertig, O.; Hamburger, M.; Atanassov, I.; Butterweck, V. Recovery of Polyphenols from Rose Oil Distillation Wastewater Using Adsorption Resins—A Pilot Study. Planta Med. 2014, 80, 1657–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabahi, Z.; Farmani, F.; Mousavinoor, E.; Moein, M. Valorization of Waste Water of Rosa damascena Oil Distillation Process by Ion Exchange Chromatography. Sci. World J. 2020, 2020, 5409493. [Google Scholar] [CrossRef] [PubMed]
- Slavov, A.; Kiyohara, H.; Yamada, H. Immunomodulating pectic polysaccharides from waste rose petals of Rosa damascena Mill. Int. J. Biol. Macromol. 2013, 59, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, E.-S.; Bazaid, S.A.; Salih, A.; Sabra, A.N.A. Total phenolic, in vitro antioxidant activity and safety assessment (Acute, sub-chronic and chronic toxicity) of industrial Taif rose water by-product in mice. Der. Pharm. Lett. 2015, 7, 251–259. [Google Scholar]
- Solimine, J.; Garo, E.; Wedler, J.; Rusanov, K.; Fertig, O.; Hamburger, M.; Atanassov, I.; Butterweck, V. Tyrosinase inhibitory constituents from a polyphenol enriched fraction of rose oil distillation wastewater. Fitoterapia 2016, 108, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, I.; Krastev, L.; Petkova, N.; Yantcheva, N.; Nenov, N.; Krachmarov, A.; Atanasova, A.; Slavov, A. Valorization of cacao and rose waste for preparation of liqueurs. Food Sci. Appl. Biotechnol. 2019, 2, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.; Fattahi, M. The study of active compounds and antioxidant activity of waste residues obtained during Essential oil and Rose water production in 24 Damask Rose Populations of East and West Azerbaijan: By-product. J. Plant Res. (Iran. J. Biol.) 2021, 34, 945–956. [Google Scholar]
- Wedler, J.; Rusanov, K.; Atanassov, I.; Butterweck, V. A Polyphenol-Enriched Fraction of Rose Oil Distillation Wastewater Inhibits Cell Proliferation, Migration and TNF-α-Induced VEGF Secretion in Human Immortalized Keratinocytes. Planta Medica 2016, 82, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, M.; Ivanov, G.; Mihalev, K.; Slavchev, A.; Ivanova, I.; Vlaseva, R. Investigation of antimicrobial activity of polyphenol-enriched extracts against probiotic lactic acid bacteria. Food Sci. Appl. Biotechnol. 2019, 2, 67–73. [Google Scholar] [CrossRef]
- Slavov, A.; Vasileva, I.; Nenov, N.; Stefanov, L.; Shikov, V.; Mihalev, K.; Stoyanova, A. Wastes from the rose oil industry–approaches for valorization and recycling. Inf. Bull. Bulg. Natl. Assoc. Essent. Oils Perfum. Cosmet. 2018, 76, 39–45. [Google Scholar]
- Chochkov, R.; Vasileva, I.; Ivanova, P.; Slavov, A. Effect of industrial Rosa Damascena Mill Waste in Pastry Biscuits. In Proceedings of the 87 International Scientific Conference of Young Scientist and Students “Youth Scientific Achievements to the 21st Century Nutrition Problem Solution”, Kyiv, Ukraine, 7–8 April 2021. [Google Scholar]
- Mollov, P.; Mihalev, K.; Shikov, V.; Yoncheva, N.; Karagyozov, V. Colour stability improvement of strawberry beverage by fortification with polyphenolic copigments naturally occurring in rose petals. Innov. Food Sci. Emerg. Technol. 2007, 8, 318–321. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Khalid, R.; Hanif, M.A. Dynamic biosorption of Zn(II) and Cu(II) using pretreated Rosa gruss an teplitz (red rose) distillation sludge. Chem. Eng. J. 2009, 148, 434–443. [Google Scholar] [CrossRef]
- Ansari, T.M.; Hanif, M.A.; Mahmood, A.; Ijaz, U.; Khan, M.A.; Nadeem, R.; Ali, M. Immobilization of rosewaste biomass for uptake of Pb(II) from aqueous solutions. Biotechnol. Res. Int. 2011, 2011, 685023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaboyaci, M.; Karaboyacı, M. Recycling of rose wastes for use in natural plant dye and industrial applications. J. Text. Inst. 2014, 105, 1160–1166. [Google Scholar] [CrossRef]
- Eren, E.; Gok, E.C.; Seyhan, B.N.; Maslakci, N.N.; Oksuz, A.U. Evaluation of Anthocyanin, a Rose Residue Extract, for Use in Dye-Sensitized Solar Cell. Asian J. Chem. 2015, 27, 3745–3748. [Google Scholar] [CrossRef]
- Rabbani, D.; Mahmoudkas, N.; Mehdizad, F.; Shaterian, M. Green Approach to Wastewater Treatment by Application of Rosa damascena Waste as Nano-Biosorbent. J. Environ. Sci. Technol. 2016, 9, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Georgieva, A.; Ilieva, Y.; Kokanova-Nedialkova, Z.; Zaharieva, M.M.; Nedialkov, P.; Dobreva, A.; Kroumov, A.; Najdenski, H.; Mileva, M. Redox-Modulating Capacity and Antineoplastic Activity of Wastewater Obtained from the Distillation of the Essential Oils of Four Bulgarian Oil-Bearing Roses. Antioxidants 2021, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Jovtchev, G.; Stergios, M.; Schubert, I. A comparison of N-methyl-N-nitrosourea-induced chromatid aberrations and micronuclei in barley meristems using FISH techniques. Mutat. Res. Toxicol. Environ. Mutagen. 2002, 517, 47–51. [Google Scholar] [CrossRef]
- Evans, H. Handbook of Mutagenicity Test Procedures; Kilbey, B., Legator, M., Nicols, W., Ramel, C., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1984; pp. 405–427. [Google Scholar]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluska, M.; Juszczak, M.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol and Its Glycoside Derivatives as Modulators of Etoposide Activity in HL-60 Cells. Int. J. Mol. Sci. 2021, 22, 3520. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Barcelos, G.; Grotto, D.; Angeli, J.P.F.; Serpeloni, J.M.; Rocha, B.; Bastos, J.K.; Barbosa, F., Jr. Evaluation of Antigenotoxic Effects of Plant Flavonoids Quercetin and Rutin on HepG2 Cells. Phytother. Res. 2011, 25, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Volti, G.L.; Cardile, V.; Abraham, N.G.; Sorrenti, V. Effects of Ellagic Acid on Angiogenic Factors in Prostate Cancer Cells. Cancers 2013, 5, 726–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and Kaempferol Rhamnosides with Depigmenting and Anti-Inflammatory Properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Montaño, J.M.; Burgos-Morón, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Jovtchev, G.; Gateva, S.; Stankov, A. Lilium compounds kaempferol and jatropham can modulate cytotoxic and genotoxic effects of radiomimetic zeocin in plants and human lymphocytesIn vitro. Environ. Toxicol. 2016, 31, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Juszczak, M.; Wysokinski, D.; Żuchowski, J.; Stochmal, A.; Wozniak, K. Kaempferol derivatives isolated from Lens culinaris Medik. reduce DNA damage induced by etoposide in peripheral blood mononuclear cells. Toxicol. Res. 2019, 8, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Iovine, C.; Mottola, F.; Santonastaso, M.; Finelli, R.; Agarwal, A.; Rocco, L. In vitro ameliorative effects of ellagic acid on vitality, motility and DNA quality in human spermatozoa. Mol. Reprod. Dev. 2021, 88, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Mottola, F.; Finelli, R.; Iovine, C.; Carannante, M.; Santonastaso, M.; Rocco, L. Anti-Genotoxicity Evaluation of Ellagic Acid and Curcumin—An In Vitro Study on Zebrafish Blood Cells. Appl. Sci. 2021, 11, 8142. [Google Scholar] [CrossRef]
- Girish, C.; Koner, B.C.; Jayanthi, S.; Rao, K.R.; Rajesh, B.; Pradhan, S.C. Hepatoprotective activity of picroliv, curcumin and ellagic acid compared to silymarin on paracetamol induced liver toxicity in mice. Fundam. Clin. Pharmacol. 2009, 23, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Khanduja, K.L.; Gandhi, R.K.; Pathania, V.; Syal, N. Prevention of N-nitrosodiethylamine-induced lung tumourigenesis by ellagic- acid and quercetin in mice. Food Chem. Toxicol. 1999, 37, 313–318. [Google Scholar] [CrossRef]
- Davis, W.L.; Matthew, S.B. Antioxidants and cancer III: Quercetin. Altern. Med. Rev. 2000, 5, 196–208. [Google Scholar]
- Shirahata, S.; Murakami, H.; Nishiyama, K.; Yamada, K.; Nonaka, G.; Nishioka, I.; Omura, H. DNA breakage by flavan-3-ols and procyanidins in the presence of cupric ion. J. Agric. Food Chem. 1989, 37, 299–303. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Nowak, A. Cytotoxic and DNA-Damaging Effects of Aronia melanocarpa, Cornus mas, and Chaenomeles superba Leaf Extracts on the Human Colon Adenocarcinoma Cell Line Caco-2. Antioxidants 2020, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Black, A.K.; McFarland, R.D.; Grisham, J.W.; Smith, G.J. Cell cycle perturbation and cell death after exposure of a human lymphoblastoid cell strain to N-methyl-N’-nitro-N-nitrosoguanidine. Am. J. Pathol. 1989, 134, 53–61. [Google Scholar] [PubMed]
- Sancar, A.; Lindsey-Boltz, L.A.; Ünsal-Kaçmaz, K.; Linn, S. Molecular Mechanisms of Mammalian DNA Repair and the DNA Damage Checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drabløs, F.; Feyzi, E.; Aas, P.A.; Vaagbø, C.B.; Kavli, B.; Bratlie, M.S.; Peña-Diaz, J.; Otterlei, M.; Slupphaug, G.; Krokan, H.E. Alkylation damage in DNA and RNA—Repair mechanisms and medical significance. DNA Repair 2004, 3, 1389–1407. [Google Scholar] [CrossRef] [PubMed]













| Treatment Variants | Hot | Spot | Segments | |||||
|---|---|---|---|---|---|---|---|---|
| Non-Hot Spot Segments | Chr.2 Segm.12  | Chr.4 3 * Segm.21  | Chr.6 Segm.37  | Chr.6 Segm.39  | Chr.7 1 * Segm.44  | Chr.7 1 * Segm.48  | ||
| 1 | Untreated control | 100% | - | - | - | - | - | - |
| 2 | R. alba ww 6% 1 h | 100% | - | - | - | - | - | - |
| R. damascena ww 6% 1 h | 85.7% | - | - | - | - | - | 14.3% | |
| 3 | R. alba ww 14% 1 h | 100% | - | - | - | - | - | - |
| R. damascena ww 14% 1 h | 100% | - | - | - | - | - | - | |
| 4 | R. alba ww 20% 1 h | 73.7% | - | - | 15.8% | 10.5% | - | - |
| R. damascena ww 20% 1 h | 87.3% | - | - | - | - | - | 12.7% | |
| 5 | R. alba ww 6% 4 h | 100% | - | - | - | - | - | - |
| R. damascena ww 6% 4 h | 100% | - | - | - | - | - | - | |
| 6 | R. alba ww 14% 4 h | 100% | - | - | - | - | - | - |
| R. damascena ww 14% 4 h | 87.1% | - | - | - | - | - | 12.9% | |
| 7 | R. alba ww 20% 4 h | 86.4% | - | - | 13.6% | - | - | - |
| R. damascena ww 20% 4 h | 100% | - | - | - | - | - | - | |
| 8 | MNNG 50 µg/mL | 52.2% | 6.8% | 5.3% | 7.8% | 7.1% | 9.9% | 10.6% |
| Treatment variants | Hot | Spot | Segments | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-hot spot segments | Chr.2 segm.12  | Chr.43 * segm.21  | Chr.43 * segm.24  | Chr.6 segm.37  | Chr.6 segm.39  | Chr.71 * segm.44  | Chr.71 * segm.48  | ||
| 1 | Untreated control | 100% | - | - | - | - | - | - | - |
| 2 | R. alba ww 20%-MNNG50 | 80.4% | - | - | 7.6% | - | - | 12.0% | - |
| R. damacsena ww 20%-MNNG50 | 77.2% | - | - | - | - | - | 12.3% | 10.5% | |
| 3 | R. alba ww 20%→4 h→MNNG50 | 77.1% | - | - | 10.9% | - | - | 12.0% | - |
| R. damascena ww 20%→4 h→MNNG50 | 86.0% | - | 6.5% | - | - | - | - | 7.5% | |
| 4 | MNNG 50 µg/ml | 52.2% | 6.8% | 5.3% | - | 7.8% | 7.1% | 9.9% | 10.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gateva, S.; Jovtchev, G.; Angelova, T.; Dobreva, A.; Mileva, M. The Anti-Genotoxic Activity of Wastewaters Produced after Water-Steam Distillation of Bulgarian Rosa damascena Mill. and Rosa alba L. Essential Oils. Life 2022, 12, 455. https://doi.org/10.3390/life12030455
Gateva S, Jovtchev G, Angelova T, Dobreva A, Mileva M. The Anti-Genotoxic Activity of Wastewaters Produced after Water-Steam Distillation of Bulgarian Rosa damascena Mill. and Rosa alba L. Essential Oils. Life. 2022; 12(3):455. https://doi.org/10.3390/life12030455
Chicago/Turabian StyleGateva, Svetla, Gabriele Jovtchev, Tsveta Angelova, Ana Dobreva, and Milka Mileva. 2022. "The Anti-Genotoxic Activity of Wastewaters Produced after Water-Steam Distillation of Bulgarian Rosa damascena Mill. and Rosa alba L. Essential Oils" Life 12, no. 3: 455. https://doi.org/10.3390/life12030455
APA StyleGateva, S., Jovtchev, G., Angelova, T., Dobreva, A., & Mileva, M. (2022). The Anti-Genotoxic Activity of Wastewaters Produced after Water-Steam Distillation of Bulgarian Rosa damascena Mill. and Rosa alba L. Essential Oils. Life, 12(3), 455. https://doi.org/10.3390/life12030455