Modulation by Estradiol of L-Dopa-Induced Dyskinesia in a Rat Model of Post-Menopausal Hemiparkinsonism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
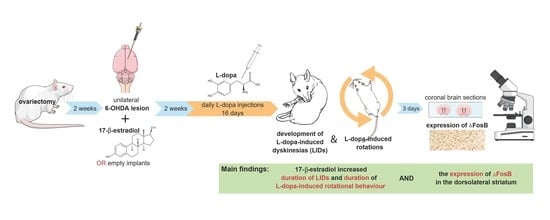
2.2. Experimental Design
2.3. Behavioural Testing
2.3.1. L-Dopa-Induced Dyskinesias (LIDs)
2.3.2. Rotation Test (RT)
2.3.3. Forepaw Adjusted Step Test (FAS Test)
2.4. Immunohistochemistry
2.4.1. Chromogenic Immunohistochemical Labelling of TH and ΔFosB
2.4.2. Densitometric Analysis of TH Staining in SN
2.4.3. Automated Counting of ΔFosB-ir Nuclei
2.5. Serum Levels of 17-β-Estradiol
2.6. Statistical Analysis
3. Results
3.1. Behaviour Analysis
3.1.1. LID Testing
3.1.2. Rotation Test
3.1.3. FAS Test
3.2. Body Weight
3.3. Serum E2 Levels
3.4. Immunohistochemical Analysis
3.4.1. Loss of Dopaminergic Neurons in SN
3.4.2. Expression of ΔFosB
4. Discussion
4.1. AIMs
4.2. Rotation Test
4.3. Expression of ΔFosB
4.4. The FAS Test
4.5. Heterogeneity of Data
4.6. Weight Loss
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flisar, D.; Trošt, M.; Pirtošek, Z. Možnosti zdravljenja napredovale Parkinsonove bolezni. Zdrav. Vestn. 2016, 85, 10. [Google Scholar] [CrossRef] [Green Version]
- Ungerstedt, U.; Arbuthnott, G.W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970, 24, 485–493. [Google Scholar] [CrossRef]
- Gerfen, C.R. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum animal model of Parkinson’s disease. Neuroscientist 2003, 9, 455–462. [Google Scholar] [CrossRef]
- Gnanalingham, K.K.; Robertson, R.G. The effects of chronic continuous versus intermittent levodopa treatments on striatal and extrastriatal D1 and D2 dopamine receptors and dopamine uptake sites in the 6-hydroxydopamine lesioned rat--an autoradiographic study. Brain Res. 1994, 640, 185–194. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Nicoletti, A.; Arabia, G.; Pugliese, P.; Nicoletti, G.; Torchia, G.; Condino, F.; Morgante, L.; Quattrone, A.; Zappia, M. Hormonal replacement therapy in women with Parkinson disease and levodopa-induced dyskinesia: A crossover trial. Clin. Neuropharmacol. 2007, 30, 276–280. [Google Scholar] [CrossRef]
- Blanchet, P.J.; Fang, J.; Hyland, K.; Arnold, L.A.; Mouradian, M.M.; Chase, T.N. Short-term effects of high-dose 17beta-estradiol in postmenopausal PD patients: A crossover study. Neurology 1999, 53, 91–95. [Google Scholar] [CrossRef]
- Gomez-Mancilla, B.; Bedard, P.J. Effect of estrogen and progesterone on L-dopa induced dyskinesia in MPTP-treated monkeys. Neurosci. Lett. 1992, 135, 129–132. [Google Scholar] [CrossRef]
- Smith, C.P.; Oh, J.D.; Bibbiani, F.; Collins, M.A.; Avila, I.; Chase, T.N. Tamoxifen effect on L-DOPA induced response complications in parkinsonian rats and primates. Neuropharmacology 2007, 52, 515–526. [Google Scholar] [CrossRef]
- Ungerstedt, U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol. Scand. Suppl. 1971, 367, 69–93. [Google Scholar] [CrossRef] [PubMed]
- Glavan, G. Intermittent L-DOPA treatment differentially alters synaptotagmin 4 and 7 gene expression in the striatum of hemiparkinsonian rats. Brain Res. 2008, 1236, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.A.; Lundblad, M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson’s disease in rats and mice. Curr. Protoc. Neurosci. 2007, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Valdez, A.L.; Rodríguez-Lara, V.; Anaya-Martínez, V.; Ordóñez-Librado, J.L.; Sanchez-Betancourt, J.; Montiel-Flores, E.; Reynoso-Erazo, L.; Tron-Alvarez, R.; Aley-Medina, P.; Espinosa-Villanueva, J.; et al. Differences Between Intact and Ovariectomized Hemiparkinsonian Rats in Response to L-DOPA, Melatonin, and L-DOPA/Melatonin Coadministration on Motor Behavior and Cytological Alterations. In Sex Hormones in Neurodegenerative Processes and Diseases; IntechOpen: London, UK, 2018; pp. 171–205. [Google Scholar]
- Gillies, G.E.; Murray, H.E.; Dexter, D.; McArthur, S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2004, 78, 513–522. [Google Scholar] [CrossRef]
- Ebihara, K.; Ishida, Y.; Takeda, R.; Abe, H.; Matsuo, H.; Kawai, K.; Magata, Y.; Nishimori, T. Differential expression of FosB, c-Fos, and Zif268 in forebrain regions after acute or chronic L-DOPA treatment in a rat model of Parkinson’s disease. Neurosci. Lett. 2011, 496, 90–94. [Google Scholar] [CrossRef]
- Perrotti, L.I.; Hadeishi, Y.; Ulery, P.G.; Barrot, M.; Monteggia, L.; Duman, R.S.; Nestler, E.J. Induction of deltaFosB in reward-related brain structures after chronic stress. J. Neurosci. 2004, 24, 10594–10602. [Google Scholar] [CrossRef] [Green Version]
- Pavon, N.; Martin, A.B.; Mendialdua, A.; Moratalla, R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol. Psychiatry 2006, 59, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.; Hilbertson, A.; Cenci, M.A. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol. Dis. 1999, 6, 461–474. [Google Scholar] [CrossRef] [Green Version]
- Cenci, M.A.; Tranberg, A.; Andersson, M.; Hilbertson, A. Changes in the regional and compartmental distribution of FosB- and JunB-like immunoreactivity induced in the dopamine-denervated rat striatum by acute or chronic L-dopa treatment. Neuroscience 1999, 94, 515–527. [Google Scholar] [CrossRef]
- Lindgren, H.S.; Rylander, D.; Iderberg, H.; Andersson, M.; O’Sullivan, S.S.; Williams, D.R.; Lees, A.J.; Cenci, M.A. Putaminal upregulation of FosB/DeltaFosB-like immunoreactivity in Parkinson’s disease patients with dyskinesia. J. Parkinsons Dis. 2011, 1, 347–357. [Google Scholar] [CrossRef]
- Girasole, A.E.; Lum, M.Y.; Nathaniel, D.; Bair-Marshall, C.J.; Guenthner, C.J.; Luo, L.; Kreitzer, A.C.; Nelson, A.B. A Subpopulation of Striatal Neurons Mediates Levodopa-Induced Dyskinesia. Neuron 2018, 97, 787–795.e6. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, D.; Conti, M.M.; Ostock, C.Y.; George, J.A.; Goldenberg, A.A.; Melikhov-Sosin, M.; Nuss, E.E.; Bishop, C. The Role of Primary Motor Cortex (M1) Glutamate and GABA Signaling in l-DOPA-Induced Dyskinesia in Parkinsonian Rats. J. Neurosci. 2016, 36, 9873–9887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, M.; Nikkhah, G.; Bentlage, C.; Bjorklund, A. Forelimb akinesia in the rat Parkinson model: Differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 1995, 15, 3863–3875. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Wachtel, S.R.; Young, D.; Kang, U.J. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: Studies on medial forebrain bundle and striatal lesions. Neuroscience 1999, 88, 617–628. [Google Scholar] [CrossRef]
- Zorović, M.; Kolmančič, K.; Živin, M. Effects of L-dopa on expression of prolactin and synaptotagmin IV in 17-beta-estradiol-induced prolactinomas of ovariectomized hemiparkinsonian rats. Bosn. J. Basic Med. Sci. 2021, 21, 702–711. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2007; 456p. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Murray, H.E.; Pillai, A.V.; McArthur, S.R.; Razvi, N.; Datla, K.; Dexter, D.; Gillies, G. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: Differential actions of estrogen in males and females. Neuroscience 2003, 116, 213–222. [Google Scholar] [CrossRef]
- Cordellini, M.F.; Piazzetta, G.; Pinto, K.C.; Delattre, A.M.; Matheussi, F.; Carolino, R.O.G.; Szawka, R.E.; Anselmo-Franci, J.A.; Ferraz, A.C. Effect of different doses of estrogen on the nigrostriatal dopaminergic system in two 6-hydroxydopamine-induced lesion models of Parkinson’s disease. Neurochem Res 2011, 36, 955–961. [Google Scholar] [CrossRef]
- Gajjar, T.M.; Anderson, L.I.; Dluzen, D.E. Acute effects of estrogen upon methamphetamine induced neurotoxicity of the nigrostriatal dopaminergic system. J. Neural Transm. 2003, 110, 1215–1224. [Google Scholar] [CrossRef]
- Smith, G.A.; Heuer, A.; Dunnett, S.B.; Lane, E.L. Unilateral nigrostriatal 6-hydroxydopamine lesions in mice II: Predicting l-DOPA-induced dyskinesia. Behav. Brain Res. 2012, 226, 281–292. [Google Scholar] [CrossRef]
- van der Kooy, D.; Kuypers, H.G. Fluorescent retrograde double labeling: Axonal branching in the ascending raphe and nigral projections. Science 1979, 204, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Kirik, D.; Bjorklund, A.; Cenci, M.A. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson’s disease: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 2002, 10, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Francardo, V.; Recchia, A.; Popovic, N.; Andersson, D.; Nissbrandt, H.; Cenci, M.A. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 42, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.B.; Bair-Marshall, C.; Nelson, A.B. Aberrant Striatal Activity in Parkinsonism and Levodopa-Induced Dyskinesia. Cell Rep. 2018, 23, 3438–3446.e5. [Google Scholar] [CrossRef] [PubMed]
- Fieblinger, T.; Zanetti, L.; Sebastianutto, I.; Breger, L.; Quintino, L.; Lockowandt, M.; Lundberg, C.; Cenci, M.A. Striatonigral neurons divide into two distinct morphological-physiological phenotypes after chronic L-DOPA treatment in parkinsonian rats. Sci. Rep. 2018, 8, 10068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doucet, J.P.; Nakabeppu, Y.; Bedard, P.J.; Hope, B.T.; Nestler, E.J.; Jasmin, B.J.; Chen, J.S.; Iadarola, M.J.; St-Jean, M.; Wigle, N.; et al. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum. Eur. J. Neurosci. 1996, 8, 365–381. [Google Scholar] [CrossRef]
- Haaxma, C.A.; Bloem, B.R.; Borm, G.F.; Oyen, W.J.; Leenders, K.L.; Eshuis, S.; Booij, J.; Dluzen, D.E.; Horstink, M.W.I.M. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Shukla, G.; Goyal, V.; Singh, S.; Behari, M. A case control study of women with Parkinson’s disease and their fertility characteristics. J. Neurol. Sci. 2012, 319, 135–138. [Google Scholar] [CrossRef]
- Cereda, E.; Barichella, M.; Cassani, E.; Caccialanza, R.; Pezzoli, G. Reproductive factors and clinical features of Parkinson’s disease. Parkinsonism Relat. Disord. 2013, 19, 1094–1099. [Google Scholar] [CrossRef]
- Perez, X.A.; Zhang, D.; Bordia, T.; Quik, M. Striatal D1 medium spiny neuron activation induces dyskinesias in parkinsonian mice. Mov. Disord. 2017, 32, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Mosharov, E.V.; Borgkvist, A.; Sulzer, D. Presynaptic effects of levodopa and their possible role in dyskinesia. Mov. Disord. 2015, 30, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hefti, F.; Melamed, E.; Sahakian, B.J.; Wurtman, R.J. Circling behavior in rats with partial, unilateral nigro-striatal lesions: Effect of amphetamine, apomorphine, and DOPA. Pharmacol. Biochem. Behav. 1980, 12, 185–188. [Google Scholar] [CrossRef]
- Robertson, G.S.; Robertson, H.A. Evidence that the substantia nigra is a site of action for L-DOPA. Neurosci. Lett. 1988, 89, 204–208. [Google Scholar] [CrossRef]
- Beyer, P.L.; Palarino, M.Y.; Michalek, D.; Busenbark, K.; Koller, W.C. Weight change and body composition in patients with Parkinson’s disease. J. Am. Diet Assoc. 1995, 95, 979–983. [Google Scholar] [CrossRef]
- Abbott, R.A.; Cox, M.; Markus, H.; Tomkins, A. Diet, body size and micronutrient status in Parkinson’s disease. Eur. J. Clin. Nutr. 1992, 46, 879–884. [Google Scholar] [PubMed]
- Durrieu, G.; Me, L.L.; Rascol, O.; Senard, J.M.; Rascol, A.; Montastruc, J.L. Parkinson’s disease and weight loss: A study with anthropometric and nutritional assessment. Clin. Auton. Res. 1992, 2, 153–157. [Google Scholar] [CrossRef]
- Wszolek, Z.K.; Markopoulou, K. Olfactory dysfunction in Parkinson’s disease. Clin. Neurosci. 1998, 5, 94–101. [Google Scholar]
- Edwards, L.L.; Pfeiffer, R.F.; Quigley, E.M.; Hofman, R.; Balluff, M. Gastrointestinal symptoms in Parkinson’s disease. Mov. Disord. 1991, 6, 151–156. [Google Scholar] [CrossRef]
- Markus, H.S.; Cox, M.; Tomkins, A.M. Raised resting energy expenditure in Parkinson’s disease and its relationship to muscle rigidity. Clin. Sci. 1992, 83, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Heiman, M.L. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E315–E322. [Google Scholar] [CrossRef]
- Koebele, S.V.; Bimonte-Nelson, H.A. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas 2016, 87, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortepiani, L.A.; Zhang, H.; Racusen, L.; Roberts, L.J., II; Reckelhoff, J.F. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension 2003, 41, 640–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoegh-Andersen, P.; Tanko, L.B.; Andersen, T.L.; Lundberg, C.V.; Mo, J.A.; Heegaard, A.-M.; Delaissé, J.-M.; Christgau, S. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthritis Res. Ther. 2004, 6, R169–R180. [Google Scholar] [CrossRef] [PubMed] [Green Version]












| Treatment Group | Implants | Daily Injections 1 | n |
|---|---|---|---|
| E2+L-dopa | E2 | L-dopa | 15 |
| L-dopa | Empty | L-dopa | 10 |
| E2+saline | E2 | Saline | 12 |
| Saline | Empty | Saline | 13 |
| Axial AIMs | Limb AIMs | Orolingual AIMs | ||
|---|---|---|---|---|
| amplitude | 1 | Sustained deviation of head and neck, at ~30° angle. | Tiny movements of the paw around the snout. | Twitching of facial muscles, small masticatory movements without jaw opening. |
| 2 | Sustained deviation of head and neck, angle ≤ 60°. | Movements of the whole limb up and down from the floor to the snout. | Twitching of facial muscles, noticeable masticatory movements occasional jaw opening. | |
| 3 | Sustained twisting of the head, neck, and upper trunk at an angle > 60° but ≤90°. | Large displacement of the whole limb with visible contraction of shoulder muscles, digital extension. | Movements with broad involvement of facial muscles and masticatory muscles, frequent jaw opening, occasional tongue protrusion. | |
| 4 | Sustained twisting of the head, neck, and trunk at maximal amplitude (angle > 90°), causing the rat to lose balance (from a bipedal position). | Dystonic posture with elbow extension, digital flexion into fists. | All the above muscle categories are involved to the maximal degree. | |
| duration | 0 | No dyskinesia. | ||
| 1 | Less than 30 s. | |||
| 2 | More than 30 s. | |||
| 3 | Continuous dyskinesia during the entire observation time, but is suppressible by external stimuli (hand clapping). | |||
| 4 | Continuous dyskinesia during the entire observation time that is not suppressible by external stimuli. | |||
| Treatment Group | Percentage of Neuron Loss in SN ( ± SD) | n |
|---|---|---|
| E2+L-dopa | 88.7 ± 5.8 | 15 |
| L-dopa | 89.9 ± 6.2 | 10 |
| E2+saline | 91.7 ± 6.6 | 12 |
| Saline | 87.6 ± 6.3 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolmančič, K.; Živin, M.; Zorović, M. Modulation by Estradiol of L-Dopa-Induced Dyskinesia in a Rat Model of Post-Menopausal Hemiparkinsonism. Life 2022, 12, 640. https://doi.org/10.3390/life12050640
Kolmančič K, Živin M, Zorović M. Modulation by Estradiol of L-Dopa-Induced Dyskinesia in a Rat Model of Post-Menopausal Hemiparkinsonism. Life. 2022; 12(5):640. https://doi.org/10.3390/life12050640
Chicago/Turabian StyleKolmančič, Kaja, Marko Živin, and Maja Zorović. 2022. "Modulation by Estradiol of L-Dopa-Induced Dyskinesia in a Rat Model of Post-Menopausal Hemiparkinsonism" Life 12, no. 5: 640. https://doi.org/10.3390/life12050640
APA StyleKolmančič, K., Živin, M., & Zorović, M. (2022). Modulation by Estradiol of L-Dopa-Induced Dyskinesia in a Rat Model of Post-Menopausal Hemiparkinsonism. Life, 12(5), 640. https://doi.org/10.3390/life12050640