Effects of a Combined Intradialytic Exercise Training Program and Music on Cardiac Autonomic Nervous System Activity in Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
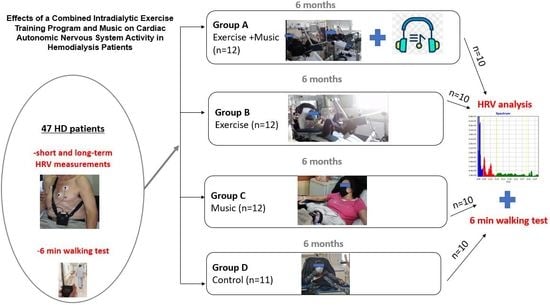
2.2. Study Design and Intervention
2.3. Functional Capacity
2.4. 24 h Measurement of HRV
2.5. Acute Short-Term Measurement of HRV
2.6. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Ambulatory 24-h Holter monitoring
3.3. Acute HRV Indices
3.4. Functional Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C.A. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant. 2019, 34, 1803–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, M.; Coppolino, G.; Faga, T.; Garofalo, C.; Serra, R.; Andreucci, M. Epidemiology of cardiovascular risk in chronic kidney disease patients: The real silent killer. Rev. Cardiovasc. Med. 2019, 20, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Salman, I.M. Cardiovascular Autonomic Dysfunction in Chronic Kidney Disease: A Comprehensive Review. Curr. Hypertens. Rep. 2015, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.P.; Jong, P.; Barry-Bianchi, S.M.; Tanaka, T.H.; Floras, J.S. Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: A systematic review. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 386–396. [Google Scholar] [CrossRef]
- Ranpuria, R.; Hall, M.; Chan, C.T.; Unruh, M. Heart rate variability (HRV) in kidney failure: Measurement and consequences of reduced HRV. Nephrol. Dial. Transpl. 2008, 23, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.H.; Tsai, T.J. Autonomic dysfunction in chronic kidney disease: An old problem in a new era. J. Formos. Med. Assoc. 2016, 115, 687–688. [Google Scholar] [CrossRef] [Green Version]
- Green, D.; Roberts, P.R.; New, D.I.; Kalra, P.A. Sudden cardiac death in hemodialysis patients: An in-depth review. Am. J. Kidney Dis. 2011, 57, 921–929. [Google Scholar] [CrossRef]
- Chan, C.T.; Levin, N.W.; Chertow, G.M.; Larive, B.; Schulman, G.; Kotanko, P. Determinants of cardiac autonomic dysfunction in ESRD. Clin. J. Am. Soc. Nephrol. 2010, 5, 1821–1827. [Google Scholar] [CrossRef] [Green Version]
- Deligiannis, A.; Kouidi, E.; Tourkantonis, A. Effects of physical training on heart rate variability in patients on hemodialysis. Am. J. Cardiol. 1999, 84, 197–202. [Google Scholar] [CrossRef]
- Kouidi, E.; Karagiannis, V.; Grekas, D.; Iakovides, A.; Kaprinis, G.; Tourkantonis, A.; Deligiannis, A. Depression, heart rate variability, and exercise training in dialysis patients. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Petraki, M.; Kouidi, E.; Grekas, D.; Deligiannis, A. Effects of exercise training during hemodialysis on cardiac baroreflex sensitivity. Clin. Nephrol. 2008, 70, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kouidi, E.J.; Grekas, D.M.; Deligiannis, A.P. Effects of exercise training on noninvasive cardiac measures in patients undergoing long-term hemodialysis: A randomized controlled trial. Am. J. Kidney Dis. 2009, 54, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Koufaki, P.; Mercer, T.H.; Naish, P.F. Effects of exercise training on aerobic and functional capacity of end-stage renal disease patients. Clin. Physiol. Funct. Imaging 2002, 22, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Orcy, R.B.; Dias, P.S.; Seus, T.L.; Barcellos, F.C.; Bohlke, M. Combined resistance and aerobic exercise is better than resistance training alone to improve functional performance of haemodialysis patients-results of a randomized controlled trial. Physiother. Res. Int. 2012, 17, 235–243. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.C.; Cicotoste, C.; Cardoso, K.; Forgiarini, L.A., Jr.; Monteiro, M.B.; Dias, A.S. Effect of exercise performed during hemodialysis: Strength versus aerobic. Ren. Fail. 2013, 35, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Ouzouni, S.; Kouidi, E.; Sioulis, A.; Grekas, D.; Deligiannis, A. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin. Rehabil. 2009, 23, 53–63. [Google Scholar] [CrossRef]
- Koelsch, S.; Jäncke, L. Music and the heart. Eur. Heart J. 2015, 36, 3043–3049. [Google Scholar] [CrossRef]
- Orini, M.; Bailón, R.; Enk, R.; Koelsch, S. A method for continuously assessing the autonomic response to music-induced emotions through HRV analysis. Med. Biol. Eng. Comput. 2010, 48, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Jia, T.; Ogawa, Y.; Miura, M.; Ito, O.; Kohzuki, M. Music Attenuated a Decrease in Parasympathetic Nervous System Activity after Exercise. PLoS ONE 2016, 11, e0148648. [Google Scholar] [CrossRef]
- Archana, R.; Mukilan, R. Beneficial Effect of Preferential Music on Exercise-Induced Changes in Heart Rate Variability. J. Clin. Diagn. Res. 2016, 10, CC09–CC11. [Google Scholar] [CrossRef] [PubMed]
- Pothoulaki, M.; Macdonald, R.A.; Flowers, P.; Stamataki, E.; Filiopoulos, V.; Stamatiadis, D.; Stathakis, C.P. An investigation of the effects of music on anxiety and pain perception in patients undergoing haemodialysis treatment. J. Health Psychol. 2008, 13, 912–920. [Google Scholar] [CrossRef]
- Melo, G.A.A.; Rodrigues, A.B.; Firmeza, M.A.; Grangeiro, A.S.M.; Oliveira, P.P.; Caetano, J. Musical intervention on anxiety and vital parameters of chronic renal patients: A randomized clinical trial. Rev. Lat. Am. Enfermagem. 2018, 26, e2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koca Kutlu, A.; Eren, A.G. Effects of music on complications during hemodialysis for chronic renal failure patients. Hemodial. Int. 2014, 18, 777–784. [Google Scholar] [CrossRef]
- Holub, C.; Lamont, M.; Lombardo, A.; Pence, T.; Schultz, G.; Tepper, S. The reliability of the six-minute walk test in patients with end-stage renal disease. Acute Care Perspect. 2002, 11, 8–11. [Google Scholar]
- American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Usui, H.; Nishida, Y. The very low-frequency band of heart rate variability represents the slow recovery component after a mental stress task. PLoS ONE 2017, 12, e0182611. [Google Scholar] [CrossRef] [Green Version]
- Vanderlei, L.C.; Silva, R.A.; Pastre, C.M.; Azevedo, F.M.; Godoy, M.F. Comparison of the Polar S810i monitor and the ECG for the analysis of heart rate variability in the time and frequency domains. Braz. J. Med. Biol. Res. 2008, 41, 854–859. [Google Scholar] [CrossRef]
- Regis da Costa, E.; Oliveira, J.; Base, L.H.; Maia, L.C.P.; Ferreira de Lima Antão, J.; de Abreu, L.C.; Oliveira, F.R.; Vanderlei, L.; Filho, C.M.; Ferreira, C. Geometric indexes of heart rate variability in healthy individuals exposed to long-term air pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 4170–4177. [Google Scholar] [CrossRef]
- Kaltsatou, A.; Flouris, A.D.; Herry, C.L.; Notley, S.R.; Macartney, M.J.; Seely, A.J.E.; Kenny, G.P. Heart rate variability in older workers during work under the Threshold Limit Values for heat exposure. Am. J. Ind. Med. 2020, 63, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Thio, C.H.L.; van Roon, A.M.; Lefrandt, J.D.; Gansevoort, R.T.; Snieder, H. Heart Rate Variability and its Relation to Chronic Kidney Disease: Results from the PREVEND Study. Psychosom. Med. 2018, 80, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deligiannis, A. Cardiac adaptations following exercise training in hemodialysis patients. Clin. Nephrol. 2004, 61 (Suppl. 1), S39–S45. [Google Scholar] [PubMed]
- Kurata, C.; Uehara, A.; Ishikawa, A. Improvement of cardiac sympathetic innervation by renal transplantation. J. Nucl. Med. 2004, 45, 1114–1120. [Google Scholar]
- Zhang, J.; Wang, N. Prognostic significance and therapeutic option of heart rate variability in chronic kidney disease. Int. Urol. Nephrol. 2014, 46, 19–25. [Google Scholar] [CrossRef]
- Besnier, F.; Labrunée, M.; Pathak, A.; Pavy-Le Traon, A.; Galès, C.; Sénard, J.M.; Guiraud, T. Exercise training-induced modification in autonomic nervous system: An update for cardiac patients. Ann. Phys. Rehabil. Med. 2017, 60, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Barroso, R.; Silva-Filho, A.C.; Dias, C.J.; Soares, N., Jr.; Mostarda, A.; Azoubel, L.A.; Melo, L.; de Mc Garcia, A.; Rodrigues, A.; Mostarda, C.T. Effect of exercise training in heart rate variability, anxiety, depression, and sleep quality in kidney recipients: A preliminary study. J. Health Psychol. 2019, 24, 299–308. [Google Scholar] [CrossRef]
- Kouidi, E.; Vergoulas, G.; Anifanti, M.; Deligiannis, A. A randomized controlled trial of exercise training on cardiovascular and autonomic function among renal transplant recipients. Nephrol. Dial. Transplant. 2013, 28, 1294–1305. [Google Scholar] [CrossRef] [Green Version]
- Bellavere, F.; Cacciatori, V.; Bacchi, E.; Gemma, M.L.; Raimondo, D.; Negri, C.; Thomaseth, K.; Muggeo, M.; Bonora, E.; Moghetti, P. Effects of aerobic or resistance exercise training on cardiovascular autonomic function of subjects with type 2 diabetes: A pilot study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 226–233. [Google Scholar] [CrossRef]
- Pagkalos, M.; Koutlianos, N.; Kouidi, E.; Pagkalos, E.; Mandroukas, K.; Deligiannis, A. Heart rate variability modifications following exercise training in type 2 diabetic patients with definite cardiac autonomic neuropathy. Br. J. Sports. Med. 2008, 42, 47–54. [Google Scholar] [CrossRef]
- Niederer, D.; Vogt, L.; Thiel, C.; Schmidt, K.; Bernhörster, M.; Lungwitz, A.; Jäger, E.; Banzer, W. Exercise effects on HRV in cancer patients. Int. J. Sports Med. 2013, 34, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Kaltsatou, A.; Hadjigeorgiou, G.M.; Grigoriou, S.S.; Karatzaferi, C.; Giannaki, C.D.; Lavdas, E.; Fotiou, D.; Kouidi, E.; Patramani, G.; Vogiatzi, C.; et al. Cardiac autonomic function during intradialytic exercise training. Postgrad. Med. 2019, 131, 539–545. [Google Scholar] [CrossRef]
- Morais, M.J.D.; de Abreu, L.C.; Santana de Oliveira, F.; Pinheiro Bezerra, I.M.; Raimundo, R.D.; Paulo Martins Silva, R.; Valenti, V.E.; Pérez-Riera, A.R. Is aerobic exercise training during hemodialysis a reliable intervention for autonomic dysfunction in individuals with chronic kidney disease? A prospective longitudinal clinical trial. J. Multidiscip. Healthc. 2019, 12, 711–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reboredo, M.; Pinheiro, V.; Neder, J.A.; Ávila, M.P.; Araujo, E.; Ribeiro, M.L.; de Mendonça, A.F.; de Melo, M.V.; Bainha, A.C.; Dondici Filho, J.; et al. Effects of aerobic training during hemodialysis on heart rate variability and left ventricular function in end-stage renal disease patients. J. Bras. Nefrol. 2010, 32, 367–373. [Google Scholar]
- Huppertz, N.; Beetham, K.S.; Howden, E.J.; Leicht, A.S.; Isbel, N.M.; Coombes, J.S. A 12-month lifestyle intervention does not improve cardiac autonomic function in patients with chronic kidney disease. Auton. Neurosci. 2020, 224, 102642. [Google Scholar] [CrossRef] [PubMed]
- Aubert, A.E.; Seps, B.; Beckers, F. Heart rate variability in athletes. Sports Med. 2003, 33, 889–919. [Google Scholar] [CrossRef]
- Raffin, J.; Barthélémy, J.C.; Dupré, C.; Pichot, V.; Berger, M.; Féasson, L.; Busso, T.; Costa, A.D.; Colvez, A.; Montuy-Coquard, C.; et al. Exercise Frequency Determines Heart Rate Variability Gains in Older People: A Meta-Analysis and Meta-Regression. Sports Med. 2019, 49, 719–729. [Google Scholar] [CrossRef]
- Carreira, M.A.; Nogueira, A.B.; Pena, F.M.; Kiuchi, M.G.; Rodrigues, R.C.; Rodrigues Rda, R.; Matos, J.P.; Lugon, J.R. Heart Rate Variability Correlates to Functional Aerobic Impairment in Hemodialysis Patients. Arq. Bras. Cardiol. 2015, 104, 493–500. [Google Scholar] [CrossRef]
- Kannankeril, P.J.; Le, F.K.; Kadish, A.H.; Goldberger, J.J. Parasympathetic effects on heart rate recovery after exercise. J. Investig. Med. 2004, 52, 394–401. [Google Scholar] [CrossRef]
- Vanoli, E.; Ferrari, G.M.; Stramba-Badiale, M.; Hull, S.S., Jr.; Foreman, R.D.; Schwartz, P.J. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ. Res. 1991, 68, 1471–1481. [Google Scholar] [CrossRef] [Green Version]
- Buch, A.N.; Coote, J.H.; Townend, J.N. Mortality, cardiac vagal control and physical training—What’s the link? Exp. Physiol. 2002, 87, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Routledge, F.S.; Campbell, T.S.; McFetridge-Durdle, J.A.; Bacon, S.L. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 2010, 26, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.J.; Smart, N.A. Exercise therapy and autonomic function in heart failure patients: A systematic review and meta-analysis. Heart Fail. Rev. 2018, 23, 91–108. [Google Scholar] [CrossRef]
- Dimitros, E.T.; Koutlianos, N.A.; Anifanti, M.; Kouidi, E.I.; Deligiannis, A.P. Comparative study of cardiorespiratory adaptations in elite basketball players of different age groups. J. Sports Med. Phys. Fit. 2021, 61, 1193–1201. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Saghazadeh, A.; Valenti, V.E.; Rezaei, N. Can music influence cardiac autonomic system? A systematic review and narrative synthesis to evaluate its impact on heart rate variability. Complement. Ther. Clin. Pract. 2020, 39, 101162. [Google Scholar] [CrossRef]
- Yamashita, S.; Iwai, K.; Akimoto, T.; Sugawara, J.; Kono, I. Effects of music during exercise on RPE, heart rate and the autonomic nervous system. J. Sports Med. Phys. Fit. 2006, 46, 425–430. [Google Scholar]
- Koufaki, P.; Kouidi, E. Current best evidence recommendations on measurement and interpretation of physical function in patients with chronic kidney disease. Sports Med. 2010, 40, 1055–1074. [Google Scholar] [CrossRef]
- Vogiatzaki, E.; Michou, V.; Liakopoulos, V.; Roumeliotis, A.; Roumeliotis, S.; Kouidi, E.; Deligiannis, A. The effect of a 6-month intradialytic exercise program on hemodialysis adequacy and body composition: A randomized controlled trial. Int. Urol. Nephrol. 2022. advance online publication. [Google Scholar] [CrossRef]
- Torres, E.; Aragoncillo, I.; Moreno, J.; Vega, A.; Abad, S.; García-Prieto, A.; Macias, N.; Hernandez, A.; Godino, M.T.; Luño, J. Exercise training during hemodialysis sessions: Physical and biochemical benefits. Ther. Apher. Dial. 2020, 24, 648–654. [Google Scholar] [CrossRef]
- Esteve Simó, V.; Junqué, A.; Fulquet, M.; Duarte, V.; Saurina, A.; Pou, M.; Moreno, F.; Carneiro, J.; Ramírez de Arellano, M. Complete low-intensity endurance training programme in haemodialysis patients: Improving the care of renal patients. Nephron Clin. Pract. 2014, 128, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.H.; Crawford, C.; Gleeson, N.P.; Naish, P.F. Low-volume exercise rehabilitation improves functional capacity and self-reported functional status of dialysis patients. Am. J. Phys. Med. Rehabil. 2002, 81, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.F.; Pereira, A.A.; Silva, W.A.; Simôes, R.; Barros Neto, J. Physical therapy during hemodialyse in patients with chronic kidney disease. J. Bras. Nefrol. 2013, 35, 170–176. [Google Scholar] [CrossRef] [PubMed]


| Group A (n = 10) | Group B (n = 10) | Group C (n = 10) | Group D (n = 10) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | |
| Sex (male/female) | 6/4 | - | 4/6 | - | 5/5 | - | 5/5 | - |
| Age (years) | 48.5 ± 15.4 | - | 50.6 ± 10.8 | - | 50.5 ± 11.5 | - | 50.2 ± 14.4 | - |
| HD duration (years) | 8.9 ± 2.8 | - | 8.8 ± 2.7 | - | 8.7 ± 2.7 | - | 8.2 ± 2.5 | - |
| CKD etiology | ||||||||
| Glomerulonephritis | 3 (30%) | - | 2 (20%) | - | 3 (30%) | - | 3 (30%) | - |
| Polycystic kidney disease | 2 (20%) | - | 2 (20%) | - | 2 (20%) | - | 3 (30%) | - |
| Nephrosclerosis | 1 (10%) | - | 2 (20%) | - | 2 (20%) | - | 1 (10%) | - |
| Vesicoureteral reflux | 2 (20%) | - | 2 (20%) | - | 1 (10%) | - | 1 (10%) | - |
| Renal ectopia | 1 (10%) | - | 0 (0%) | - | 1 (10%) | - | 0 (0%) | - |
| Interstitial nephritis | 0 (0%) | - | 1 (10%) | - | 0 (0%) | - | 1 (10%) | - |
| Unknown etiology | 1 (10%) | - | 1 (10%) | - | 1 (10%) | - | 1 (10%) | - |
| Comorbidities | ||||||||
| Cardiovascular disease | 3 (30%) | - | 2 (20%) | - | 3 (30%) | - | 2 (20%) | - |
| Hypertension | 7 (70%) | - | 8 (80%) | - | 7 (70%) | - | 8 (80%) | - |
| Hb (gr/dL) | 11.7 ± 2.3 | 11.1 ± 0.1 | 11.1 ± 7.8 | 11.6 ± 1.9 | 11.8 ± 1.4 | 12.0 ± 0.9 | 11.6 ± 3.1 | 11.7 ± 1.8 |
| Fe (μg%) | 88.9 ± 28.3 | 89.2 ± 28.3 | 90.5 ± 23.0 | 90.2 ± 25.7 | 89.7 ± 24.1 | 89.0 ± 29.7 | 90.5 ± 13.2 | 91.0 ± 11.2 |
| WBC (/mm3) | 5698 ± 1049 | 5670 ± 1730 | 5700 ± 1111 | 5428 ± 1140 | 5688 ± 1092 | 5513 ± 1420 | 5702 ± 1348 | 5700 ± 1150 |
| Glucose (mg%) | 98.2 ± 13.9 | 97.8 ± 14.7 | 98.0 ± 12.8 | 94.7 ± 16.3 | 98.2 ± 14.2 | 98.9 ± 15.3 | 97.3 ± 12.7 | 97.2 ± 13.3 |
| Urea (mg%) | 138.1 ± 17.8 | 138.3 ± 20.8 | 141.3 ± 20.3 | 140.0 ± 30.0 | 139.3 ± 20.8 | 139.1 ± 14.1 | 140.3 ± 21.4 | 141.3 ± 27.0 |
| Uric acid (mg%) | 5.8 ± 1.9 | 6.4 ± 1.2 | 5.8 ± 1.2 | 5.8 ± 1.0 | 5.8 ± 1.2 | 5.8 ± 1.1 | 5.8 ± 1.3 | 5.9 ± 1.3 |
| Cr (mg%) | 9.9 ± 2.0 | 9.9 ± 2.2 | 10.1 ± 1.0 | 10.2 ± 2.0 | 9.9 ± 1.0 | 9.9 ± 1.1 | 10.0 ± 1.8 | 10.1 ± 1.9 |
| K (mEq/L) | 5.6 ± 1.0 | 5.5 ± 0.8 | 5.6 ± 0.9 | 5.7 ± 1.1 | 5.6 ± 2.1 | 5.6 ± 1.9 | 5.5 ± 1.8 | 5.6 ± 0.1 |
| Na (mEq/L) | 135.3 ± 4.3 | 136.1 ± 2.1 | 138.2 ± 1.8 | 138.2 ± 5.0 | 136.4 ± 4.3 | 137.3 ± 3.0 | 137.5 ± 2.4 | 138.6 ± 1.2 |
| Ca (mg%) | 9.1 ± 0.9 | 9.1 ± 0.5 | 9.0 ± 4.2 | 8.9 ± 0.9 | 9.1 ± 0.7 | 9.1 ± 1.3 | 9.0 ± 4.7 | 9.0 ± 1.1 |
| P (mg%) | 6.1 ± 0.6 | 6.1 ± 1.9 | 6.0 ± 2.5 | 6.0 ± 1.3 | 6.1 ± 1.6 | 6.0 ± 1.3 | 6.0 ± 4.4 | 6.0 ± 1.3 |
| Group A | Group B | Group C | Group D | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | ||
| HR | Ambulatory | 79.5 ± 8.8 | 73.5 ± 10.9 * | 80.3 ± 5.1 | 75.7 ± 6.0 * | 81.6 ± 6.4 | 77.3 ± 9.4 * | 74.6 ± 7.1 | 73.8 ± 7.4 |
| Resting | 79.6 ± 10.0 | 73.7 ± 12.8 * | 85.6 ± 10.8 | 80.7 ± 12.9 * | 87.7 ± 10.9 | 84.0 ± 12.0 * | 82.2 ± 5.3 | 81.9 ± 4.1 | |
| SDNN | Ambulatory | 82.4 ± 2.2 | 95.1 ± 3.1 *,+ | 81.7 ± 10.9 | 96.7 ± 7.9 *,+ | 86.1 ± 3.0 | 91.6 ± 4.9 *,+ | 84.9 ± 2.1 | 84.0 ± 3.6 |
| Acute | 65.4 ± 7.0 # | 85.7 ± 9.0 *,#,$,+ | 57.0 ± 4.6 | 73.0 ± 4.1 *,+ | 63.7 ± 6.8 | 71.5 ± 7.4 *,+ | 58.3 ± 5.2 | 59.0 ± 4.6 | |
| rMSSD | Ambulatory | 20.2 ± 2.6 | 27.4 ± 3.7 *,+ | 20.5 ± 2.2 | 27.9 ± 5.6 *,+ | 21.0 ± 3.5 | 24.2 ± 2.8 *,+ | 21.1 ± 2.4 | 18.6 ± 2.3 * |
| Acute | 36.7 ± 4.4 #,$,+ | 49.8 ± 5.2 * | 44.5 ± 3.4 + | 53.3 ± 3.0 * | 48.5 ± 2.6 | 52.5 ± 2.3 * | 52.8 ± 3.5 | 50.1 ± 4.2 | |
| pNN50 | Ambulatory | 0.7 ± 0.1 | 1.7 ± 0.3 *,#,$,+ | 0.8 ± 0.2 | 1.4 ± 0.2 *,$,+ | 0.7 ± 0.1 | 0.9 ± 0.2 * | 0.7 ± 0.1 | 0.7 ± 0.1 |
| Acute | 2.3 ± 0.2 | 2.9 ± 0.3 *,$,+ | 2.2 ± 0.3 | 2.6 ± 0.3 *,+ | 2.3 ± 0.2 | 2.4 ± 0.1 * | 2.3 ± 0.2 | 2.2 ± 0.2 * | |
| LFnu | Ambulatory | 76.6 ± 4.1 + | 69.2 ± 3.7 * | 77.4 ± 3.2 | 71.2 ± 2.8 * | 73.0 ± 3.2 | 73.5 ± 3.4 | 69.4 ± 5.7 | 70.3 ± 5.1 |
| Acute | 39.9 ± 7.9 | 35.7 ± 7.5 * | 40.4 ± 7.0 | 38.6 ± 7.2 * | 40.9 ± 4.5 | 42.1 ± 5.0 | 33.9 ± 4.3 | 36.4 ± 4.3 * | |
| HFnu | Ambulatory | 37.2 ± 11.1 | 31.7 ± 10.9 | 40.0 ± 8.4 $,+ | 30.4 ± 12.6 * | 28.0 ± 9.7 | 25.2 ± 10.4 | 28.4 ± 8.2 | 27.1 ± 7.9 |
| Acute | 62.9 ± 9.3 | 61.0 ± 11.0 | 60.8 ± 8.7 | 60.7 ± 6.9 | 54.3 ± 7.6 | 55.0 ± 8.0 | 54.2 ± 6.1 | 55.8 ± 6.7 * | |
| LFnu/HFn | Ambulatory | 2.2 ± 0.7 | 2.5 ± 1.0 | 2.0 ± 0.4 | 2.7 ± 1.0 | 2.9 ± 1.0 | 3.3 ± 1.1 | 2.7 ± 0.9 | 2.8 ± 0.9 |
| Acute | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.1 | |
| VLF | Ambulatory | 228.5 ± 93.3 | 189.6 ± 54.5 | 233.1 ± 50.3 | 196.3 ± 29.5 * | 174.7 ± 51.4 | 174.6 ± 54.4 | 192.0 ± 28.3 | 186.6 ± 29.6 |
| Acute | 22.6 ± 4.7 | 27.4 ± 5.2 * | 29.2 ± 6.4 | 32.3 ± 5.9 + | 25.1 ± 4.2 | 26.4 ± 4.9 | 24.7 ± 7.3 | 25.1 ± 6.3 | |
| TINN | Acute | 362.8 ± 163.3 | 418.0 ± 128.2 | 450.0 ± 103.7 | 509.1 ± 128.8 * | 456.7 ± 123.0 | 468.0 ± 118.8 * | 463.7 ± 111.4 | 434.6 ± 89.1 |
| SD1 | Acute | 48.6 ± 5.6 | 53.5 ± 5.2 * | 54.3 ± 9.5 | 63.1 ± 8.4 *,+ | 56.3 ± 10.5 | 54.3 ± 11.1 | 51.6 ± 7.1 | 50.7 ± 6.5 |
| SD2 | Acute | 57.3 ± 4.3 | 60.3 ± 5.6 | 54.7 ± 5.6 | 60.6 ± 6.4 * | 60.3 ± 10.4 | 57.8 ± 8.6 | 53.9 ± 5.6 | 53.4 ± 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsiou, M.; Dimitros, E.; Roumeliotis, S.; Liakopoulos, V.; Kouidi, E.; Deligiannis, A. Effects of a Combined Intradialytic Exercise Training Program and Music on Cardiac Autonomic Nervous System Activity in Hemodialysis Patients. Life 2022, 12, 1276. https://doi.org/10.3390/life12081276
Mitsiou M, Dimitros E, Roumeliotis S, Liakopoulos V, Kouidi E, Deligiannis A. Effects of a Combined Intradialytic Exercise Training Program and Music on Cardiac Autonomic Nervous System Activity in Hemodialysis Patients. Life. 2022; 12(8):1276. https://doi.org/10.3390/life12081276
Chicago/Turabian StyleMitsiou, Maria, Eleftherios Dimitros, Stefanos Roumeliotis, Vassilios Liakopoulos, Evangelia Kouidi, and Asterios Deligiannis. 2022. "Effects of a Combined Intradialytic Exercise Training Program and Music on Cardiac Autonomic Nervous System Activity in Hemodialysis Patients" Life 12, no. 8: 1276. https://doi.org/10.3390/life12081276
APA StyleMitsiou, M., Dimitros, E., Roumeliotis, S., Liakopoulos, V., Kouidi, E., & Deligiannis, A. (2022). Effects of a Combined Intradialytic Exercise Training Program and Music on Cardiac Autonomic Nervous System Activity in Hemodialysis Patients. Life, 12(8), 1276. https://doi.org/10.3390/life12081276