The Role of Daily Implant-Based Multiparametric Telemonitoring in Patients with a Ventricular Assist Device
Abstract
:1. Introduction
2. Methods
3. Data Collection
4. Statistical Analysis
5. Results
5.1. Patient Characteristics
5.2. ICD-/CRT-D-Programming
5.3. VAD-Related Complications
5.4. Follow-up Data
5.5. Patient Outcomes
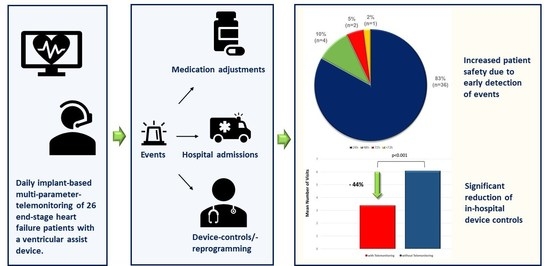
5.6. Impact of Telemonitoring on Patient Treatment and Safety
5.7. Device and Lead-Related Complications
5.8. Biventricular Pacing (BiV)
5.9. Cardiac Arrhythmias
5.10. Telemonitoring Events and Time to Detection
5.11. Effects of Telemonitoring on In-Hospital Device Controls
6. Discussion and Main Findings
6.1. Telemonitoring in HF Patients
6.1.1. Benefits in End-Stage HF
6.1.2. Impact on Time to Detection of Events and Patient Safety
6.1.3. Impact on Pre-Emptive Interventions
6.1.4. Impact on In-Hospital Device Controls
6.2. ICD Programming in VAD Patients
7. Future Perspectives and Clinical Outlook
8. Conclusions
9. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nesta, M.; Cammertoni, F.; Bruno, P.; Massetti, M. Implantable ventricular assistance systems (VAD) as a bridge to transplant or as ‘destination therapy’. Eur. Heart J. Suppl. 2021, 23 (Suppl. E), E99–E102. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, F.; Rogers, J.G. Left ventricular assist device therapy in advanced heart failure: Patient selection and outcomes. Eur. J. Heart Fail. 2017, 19, 595–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Guckel, D.; Niemann, S.; Ditzhaus, M.; Molatta, S.; Bergau, L.; Fink, T.; Sciacca, V.; El Hamriti, M.; Imnadze, G.; Steinhauer, P.; et al. Long-Term Efficacy and Impact on Mortality of Remote Magnetic Navigation Guided Catheter Ablation of Ventricular Arrhythmias. J. Clin. Med. 2021, 10, 4695. [Google Scholar] [CrossRef] [PubMed]
- Bergau, L.; Sommer, P.; Hamriti, M.E.; Morshuis, M.; Guckel, D.; Schramm, R.; Rojas, S.V.; Imnadze, G.; Gummert, J.F.; Sohns, C.; et al. Lessons learned from catheter ablation of ventricular arrhythmias in patients with a fully magnetically levitated left ventricular assist device. Clin. Res. Cardiol. 2022, 111, 574–582. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Burri, H.; Senouf, D. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace 2009, 11, 701–709. [Google Scholar] [CrossRef]
- Varma, N.; Epstein, A.E.; Irimpen, A.; Schweikert, R.; Love, C.; TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: The Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010, 122, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Saxon, L.A.; Hayes, D.L.; Gilliam, F.R.; Heidenreich, P.A.; Day, J.; Seth, M.; Meyer, T.E.; Jones, P.W.; Boehmer, J.P. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: The ALTITUDE survival study. Circulation 2010, 122, 2359–2367. [Google Scholar] [CrossRef] [Green Version]
- Landolina, M.; Perego, G.B.; Lunati, M.; Curnis, A.; Guenzati, G.; Vicentini, A.; Parati, G.; Borghi, G.; Zanaboni, P.; Valsecchi, S.; et al. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: The evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012, 125, 2985–2992. [Google Scholar] [CrossRef] [Green Version]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, T.; Koehler, K.; Wachter, R.; Moeller, V.; Zeynalova, S.; Koehler, F.; Laufs, U. Heart failure patients with atrial fibrillation benefit from remote patient management: Insights from the TIM-HF2 trial. ESC Heart Fail. 2020, 7, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Michalski, J.; Stambler, B.; Pavri, B.B.; TRUST Investigators. Superiority of automatic remote monitoring compared with in-person evaluation for scheduled ICD follow-up in the TRUST trial—Testing execution of the recommendations. Eur. Heart J. 2014, 35, 1345–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, N.; Pavri, B.B.; Stambler, B.; Michalski, J.; TRUST Investigators. Same-day discovery of implantable cardioverter defibrillator dysfunction in the TRUST remote monitoring trial: Influence of contrasting messaging systems. Europace 2013, 15, 697–703. [Google Scholar] [CrossRef]
- Hindricks, G.; Taborsky, M.; Glikson, M.; Heinrich, U.; Schumacher, B.; Katz, A.; Brachmann, J.; Lewalter, T.; Goette, A.; Block, M.; et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): A randomised controlled trial. Lancet 2014, 384, 583–590. [Google Scholar] [CrossRef]
- Geller, J.C.; Lewalter, T.; Bruun, N.E.; Taborsky, M.; Bode, F.; Nielsen, J.C.; Stellbrink, C.; Schön, S.; Mühling, H.; Oswald, H.; et al. Implant-based multi-parameter telemonitoring of patients with heart failure and a defibrillator with vs. without cardiac resynchronization therapy option: A subanalysis of the IN-TIME trial. Clin. Res. Cardiol. 2019, 108, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- García-Fernández, F.J.; Osca Asensi, J.; Romero, R.; Fernández Lozano, I.; Larrazabal, J.M.; Martínez Ferrer, J.; Ortiz, R.; Pombo, M.; Tornés, F.J.; Moradi Kolbolandi, M. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: A long-term randomized trial (RM-ALONE). Eur. Heart J. 2019, 40, 1837–1846. [Google Scholar] [CrossRef] [Green Version]
- Brenyo, A.; Rao, M.; Koneru, S.; Hallinan, W.; Shah, S.; Massey, H.T.; Chen, L.; Polonsky, B.; McNitt, S.; Huang, D.T.; et al. Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients. J. Cardiovasc. Electrophysiol. 2012, 23, 515–520. [Google Scholar] [CrossRef]
- Sohns, C.; Marrouche, N.F.; Costard-Jäckle, A.; Sossalla, S.; Bergau, L.; Schramm, R.; Fuchs, U.; Omran, H.; Rubarth, K.; Dumitrescu, D.; et al. Catheter ablation for atrial fibrillation in patients with end-stage heart failure and eligibility for heart transplantation. ESC Heart Fail. 2021, 8, 1666–1674. [Google Scholar] [CrossRef]
- El Hamriti, M.; Fox, H.; Sommer, P.; Rojas, S.V. First-in-human high-density epicardial mapping and ablation through a left anterior minithoracotomy in an LVAD patient presenting in electrical storm: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab248. [Google Scholar] [CrossRef]
- Hayes, D.L.; Boehmer, J.P.; Day, J.D.; Gilliam, F.R., 3rd; Heidenreich, P.A.; Seth, M.; Jones, P.W.; Saxon, L.A. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011, 8, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.D.; Hale, L.; Arteaga, C.; Xu, M.; Keebler, M.; Schlendorf, K.; Danter, M.; Shah, A.; Lindenfeld, J.; Ellis, C.R. Prospective Randomized Evaluation of Implantable Cardioverter-Defibrillator Programming in Patients with a Left Ventricular Assist Device. J. Am. Heart Assoc. 2018, 7, e007748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulet, J.; Massie, E.; Mondésert, B.; Lamarche, Y.; Carrier, M.; Ducharme, A. Current Review of Implantable Cardioverter Defibrillator Use in Patients with Left Ventricular Assist Device. Curr. Heart Fail. Rep. 2019, 16, 229–239. [Google Scholar] [CrossRef] [PubMed]






| Characteristics | Patients (n = 26) |
|---|---|
| Age (years) | 66 ± 11 |
| Gender, male | 23 (88%) |
| BMI (kg/m2) | 28 ± 6 |
| LVEF (%) | 22 ± 4 |
| Years since VAD-implantation | 4.10 ± 2.58 |
| VAD (DT) | 22 (85%) |
| VAD (BTT) | 4 (15%) |
| NYHA class III | 26 (100%) |
| ICM | 15 (58%) |
| DCM | 12 (46%) |
| Stroke/TIA | 12 (46%) |
| Hypertension | 23 (88%) |
| Diabetes mellitus | 11 (42%) |
| AT/AF | 21 (81%) |
| CKD | 22 (85%) |
| BB | 26 (100%) |
| Amiodarone | 22 (85%) |
| Phenprocoumon | 26 (100%) |
| AV-node ablation | 3 (12%) |
| Characteristics | Patients (n = 26) |
|---|---|
| One-chamber ICD | 6 (23%) |
| Two-chamber ICD | 5 (19%) |
| CRT-D | 15 (58%) |
| Years since device implantation | 7.83 ± 4.01 |
| RVp (%) | 67 ± 42 |
| VVI mode | 14 (54%) |
| AT/AF burden (%) | 25 ± 12 |
| PVC burden (%) | 17 ± 5 |
| VT/VF therapy activated | 24 (92%) |
| VT/VF therapy (ATP + shock) | 10 (38%) |
| VT(ATP only)/ VF therapy | 11 (42%) |
| VF therapy only | 3 (13%) |
| Tachycardia-zone reprogramming | 12 (46%) |
| Lead-problem reprogramming | 2 (8%) |
| Device downgrading | 1 (4%) |
| Characteristics | Patients |
|---|---|
| Sustained VAs | 12 (63%) |
| ATP therapy | 12 (100%) |
| Adequate shock delivery | 7 (58%) |
| AT/AF | 3 (12%) |
| Inadequate shock delivery | 3 (25%) |
| Hospital admission | 8 (67%) |
| Medication adjustment | 11 (92%) |
| Scheduled for VA-ablation | 2 (17%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guckel, D.; El Hamriti, M.; Rojas, S.V.; Fox, H.; Costard-Jäckle, A.; Gummert, J.; Fink, T.; Sciacca, V.; Isgandarova, K.; Braun, M.; et al. The Role of Daily Implant-Based Multiparametric Telemonitoring in Patients with a Ventricular Assist Device. Life 2023, 13, 38. https://doi.org/10.3390/life13010038
Guckel D, El Hamriti M, Rojas SV, Fox H, Costard-Jäckle A, Gummert J, Fink T, Sciacca V, Isgandarova K, Braun M, et al. The Role of Daily Implant-Based Multiparametric Telemonitoring in Patients with a Ventricular Assist Device. Life. 2023; 13(1):38. https://doi.org/10.3390/life13010038
Chicago/Turabian StyleGuckel, Denise, Mustapha El Hamriti, Sebastian V. Rojas, Henrik Fox, Angelika Costard-Jäckle, Jan Gummert, Thomas Fink, Vanessa Sciacca, Khuraman Isgandarova, Martin Braun, and et al. 2023. "The Role of Daily Implant-Based Multiparametric Telemonitoring in Patients with a Ventricular Assist Device" Life 13, no. 1: 38. https://doi.org/10.3390/life13010038
APA StyleGuckel, D., El Hamriti, M., Rojas, S. V., Fox, H., Costard-Jäckle, A., Gummert, J., Fink, T., Sciacca, V., Isgandarova, K., Braun, M., Khalaph, M., Imnadze, G., Schramm, R., Morshuis, M., Sommer, P., & Sohns, C. (2023). The Role of Daily Implant-Based Multiparametric Telemonitoring in Patients with a Ventricular Assist Device. Life, 13(1), 38. https://doi.org/10.3390/life13010038