Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice
Abstract
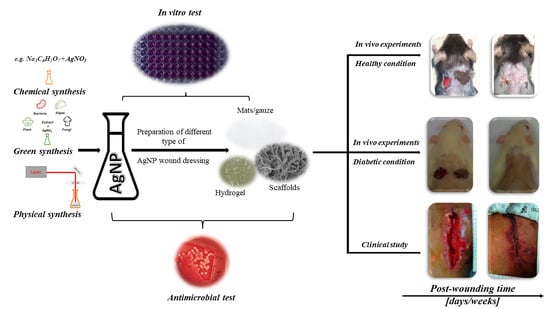
:1. Introduction
2. A Short History of Silver in Medicine
3. Antimicrobial Properties of Silver and Silver Nanoparticles
4. Mechanism of Action and Toxicity of Silver and Silver Nanoparticles
5. Biomaterials Containing Silver and Silver Nanoparticles—Experimental Studies
Diabetic Conditions
| Author | Healthy or Diabetic | Type of Wound Dressing | Healing Rate (p-Value) | Wound Status | |
|---|---|---|---|---|---|
| Control Wound | Dressed Wound | ||||
| Experimental Studies | |||||
| Niyas Ahamed et al. [55] | Healthy | Composite | p > 0.05 | Regenerated cellulose + chitosan + AgNPs | Regenerated cellulose + chitosan + AgNPs + Gentamicin |
| Qian et al. [40] | Healthy | Asymmetric wettable dressing | p < 0.05 | Gauze | Dressing based on chitosan, silk fibroin, stearic acid with a composite of exosomes and AgNPs |
| Mohseni et al. [56] | Healthy | Porous nanofibers | p < 0.05 | No dressing | Polycaprolactone/polyvinyl alcohol + AgNPs |
| Tra Thanh et al. [57] | Healthy | Multi-coated membranes | p < 0.05 | No dressing | Electrospun polycarpo-prolactone + gelatin + nanosilver |
| Konop et al. [53] | Diabetic | Fur keratin-derived powder | p < 0.05 | No dressing | Fur keratin-derived powder containing AgNPs (FKDP + AgNP) |
| Reddy et al. [61] | Diabetic | Cotton gauze Patches | p < 0.05 | Cotton gauze | Cotton gauze patches + gallocatechin and AgNPs |
| Kaur et al. [62] | Healthy and Diabetic | Different nano-formulations + gel | p < 0.05 | Saline + gel | AgNPs/ATE-Insulin/IAgNPs + gel |
| Masood et al. [63] | Diabetic | Hydrogel | p < 0.05 | No dressing | Chitosan-PEG + AgNP |
| Singla et al. [64] | Diabetic | Nano-composite dressing | p < 0.05 | Vaseline | Cellulose nanocrystals + AgNPs |
| Zhao et al. [65] | Diabetic | Hydrogel | p < 0.05 | Phosphate Buffered saline | Polydopamine decorated silver nanoparticles, polyaniline and polyvinyl alcochol |
| Shi et al. [66] | Diabetic | Hydrogel | p < 0.05 | No dressing | Maleic acid-graften dextrant and thiolated chitosan + AgNPs |
| Gaikwad et al. [67] | Healthy | Hydrogel | p < 0.05 | No dressing | Mycosynthesized Silver Nanogel |
| Banna et al. [68] | Healthy | Nano-composite gel | p < 0.05 | No dressing | Silver nano-composite gel |
| Wali et al. [69] | Healthy | Human amniotic membrane | p < 0.05 | No dressing | Colistin and AgNPs impregnated amniotic membrane |
| Kong et al. [70] | Healthy | Nanocomposite gel | p < 0.05 | No dressing | Polysaccharide riclin and AgNPs |
| Carvalho et al. [71] | Healthy | Different silver-based dressings | p < 0.05 | No dressing | Silvercel, Mepilex Ag, Aquacel Ag, Acticoat |
| Davis et al. [72] | Healthy | Hydrofiber | p < 0.05 | Polyurethane film | Anti-biofilm silver Hydrofiber |
| Liu et al. [74] | Healthy | Nanofiber | p < 0.05 | No dressing | Silver@curcumin and electrospun chitosan nanofibers |
| Type of Wound Dressing | Advantages | Disadvantages | References |
|---|---|---|---|
| Hydrocolloid | Absorbs exudate and maintains a moist environment. Thermal isolation for the wound. Pain relief properties. Easy to use in therapy | Contraindicated in infected wounds with associated clinical symptoms. The lack of experience in changing this dressing, easy to damage the skin and surrounding tissues. | [75,76,77,78] |
| Hydrogel | Maintains a moist wound healing environment, Ability to absorb wound exudate, gas permeable | Often requires a second layer of dressing, poor mechanical properties, some require frequent moistening to maintain properties | [75,76,77] |
| Scaffolds | Absorbs exudate, low adherence to the skin, biocompatible, biodegradable, gas permeable, support cell growth | Secondary dressing is necessary | [79] |
| Gauze | Cheap, easily available. The main function is to protect the wound from the external environment, easy to use, clinicians have extensive experience with this type of dressings | Sticks to the wound, make it difficult to change dressings. Does not provide moisture environment. No significant effect on wound healing. | [75,77] |
| Alginate | Absorbent, non-stick. Controlled release of substances with which they were enriched. | Contraindicated in dry wounds, often cost of therapy | [75,76,77,78] |
| Composite | Have the properties of two or more different dressings that were combined to create composite dressing. Easy to apply, provides a moist environment. | High cost of therapy | [75] |
6. Clinical Application of Wound Dressings Containing Silver and Silver Nanoparticles
| Author | Healthy or Diabetic | Type of Wound Dressing | Healing Rate (p-Value) | Wound Status | |
|---|---|---|---|---|---|
| Control Wound | Dressed Wound | ||||
| Meekul et al. [48] | Healthy | Alginate matrix | p = 0.057 | Saline solution | Alginate Silver Dressing |
| Hahn et al. [49] | Healthy | Negative-pressure wound therapy | Healing rate was not measured | Negative-pressure wound therapy | Negative-pressure wound therapy + silver |
| JiHui Chen et al. [51] | Healthy | Moist gauze | p < 0.05 | Gauze soaked in povidone iodine solution | Silver-containing dressing covered with gauze |
| Matilda Karlsson et al. [53] | Healthy | Foam or xenograft | p < 0.05 | Porcine xenograft | Silver-foam dressing |
| Akin et al. [54] | Healthy | Hydrofiber dressing | Healing rate was not measured | Gauze | Silver-containing hydrofiber dressing |
| Asgari et al. [89] | Healthy | AgNP based dressing | No statistically significant difference | Hydrocolloid | AgNP dressing |
| Berard et al. [90] | Healthy | Minimally adherent dressing | No statistically significant difference | Gauze | Minimally adherent silver dressing |
| Shi et al. [92] | Healthy | Silver sulfate dressing | Infected wound was cured | No control wound | Silver sulfate dressing |
| Miner et al. [91] | Healthy | Hydrogel sheet dressing | p < 0.05 | Petroleum-based dressing | Silver hydrogel dressing |
| Zhang et al. [94] | Diabetic | Thermoplastic polyurethane | p < 0.05 | Benzalkonium chloride coated dressing | AgNPs with thermoplastic polyurethane |
| Wang et al. [93] | Healthy | Alginate matrix | p < 0.05 | Iodoform gauze | Alginate silver dressing |
| Hurd et al. [95] | Healthy and diabetic | Silver coated polyethylene net | p < 0.05 | Gauze | Nanocrystaline silver dressing |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. (Larchmt) 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, J.M. The Story of My Life; Marion-Sims, H., Ed.; D. Appleton & Co.: New York, NY, USA, 1884. [Google Scholar]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Roe, A.L. Collosol Argentum and its Ophthalmic Uses. BMJ 1915, 1, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Guidelines for the Treatment of Neisseria Gonorrhoeae; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [Green Version]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver Nanoparticles in Therapeutics: Development of an Antimicrobial Gel Formulation for Topical Use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Govindasamy, G.A.; Mydin, R.B.S.M.N.; Effendy, W.N.F.W.E.; Sreekantan, S. Novel dual-ionic ZnO/CuO embedded in porous chitosan biopolymer for wound dressing application: Physicochemical, bactericidal, cytocompatibility and wound healing profiles. Mater. Today Commun. 2022, 33, 104545. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin, T.; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Monych, N.K.; Ghosh, S.; Turner, D.L.; Turner, R.J. Nanomaterials in Wound Healing and Infection Control. Antibiotics 2021, 10, 473. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, W.; Sun, M.; He, P.; Lv, H.; Wang, Q.; Zhang, S.; Wu, Q.; Ling, P.; Chen, S.; et al. Novel bi-layered dressing patches constructed with radially-oriented nanofibrous pattern and herbal compound-loaded hydrogel for accelerated diabetic wound healing. Appl. Mater. Today 2022, 28, 101542. [Google Scholar] [CrossRef]
- Utembe, W.; Tlotleng, N.; Kamng’ona, A. A systematic review on the effects of nanomaterials on gut microbiota. Curr. Res. Microb. Sci. 2022, 3, 100118. [Google Scholar] [CrossRef]
- Wang, P.W.; Lee, W.T.; Wu, Y.N.; Shieh, D.B. Opportunities for Nanomedicine in Clostridioides difficile Infection. Antibiotics 2021, 10, 948. [Google Scholar] [CrossRef]
- Wakshlak, R.B.K.; Pedahzur, R.; Avnir, D. Antibacterial activity of silver-killed bacteria: The “zombies” effect. Sci. Rep. 2015, 5, 9555. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Z.; Nikaido, H.; Williams, K.E. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J. Bacteriol. 1997, 179, 6127–6132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Xiang, Q.Q.; Wang, D.; Zhang, J.L.; Ding, C.Z.; Luo, X.; Tao, J.; Ling, J.; Shea, D.; Chen, L.-Q. Effect of silver nanoparticles on gill membranes of common carp: Modification of fatty acid profile, lipid peroxidation and membrane fluidity. Environ. Pollut. 2020, 256, 113504. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.; Ghanati, F.; Rezaei, A.; Bemani, E. Change of antioxidant enzymes activity of hazel (Corylus avellana L.) cells by AgNPs. Cytotechnology 2016, 68, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Quinteros, M.A.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Nadworny, P.L.; Landry, B.K.; Wang, J.; Tredget, E.E.; Burrell, R.E. Does nanocrystalline silver have a transferable effect? Wound Repair Regen. 2010, 18, 254–265. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef]
- Shin, S.H.; Ye, M.K.; Kim, H.S.; Kang, H.S. The effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int. Immunopharmacol. 2007, 7, 1813–1818. [Google Scholar] [CrossRef]
- Mani, A.K.; Seethalakshmi, S.; Gopal, V. Evaluation of In-vitro Anti-Inflammatory Activity of Silver Nanoparticles Synthesised using Piper Nigrum Extract. J. Nanomed. Nanotechnol. 2015, 6, 1000268. [Google Scholar]
- Wasef, L.G.; Shaheen, H.M.; El-Sayed, Y.S.; Shalaby, T.I.A.; Samak, D.H.; El-Hack, M.E.A.; Al-Owaimer, A.; Saadeldin, I.M.; El-Mleeh, A.; Ba-Awadh, H.; et al. Effects of Silver Nanoparticles on Burn Wound Healing in a Mouse Model. Biol. Trace Elem. Res. 2020, 193, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Siczek, K.; Zatorski, H.; Chmielowiec-Korzeniowska, A.; Kordek, R.; Tymczyna, L.; Fichna, J. Evaluation of anti-inflammatory effect of silver-coated glass beads in mice with experimentally induced colitis as a new type of treatment in inflammatory bowel disease. Pharmacol. Rep. 2017, 69, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Saeed, B.A.; Lim, V.; Yusof, N.A.; Khor, K.Z.; Rahman, H.S.; Samad, N.A. Antiangiogenic properties of nanoparticles: A systematic review. Int. J. Nanomed. 2019, 14, 5135–5146. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Lee, K.J.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef]
- Kemp, M.M.; Kumar, A.; Mousa, S.; Dyskin, E.; Yalcin, M.; Ajayan, P.; Linhardt, R.J.; Mousaet, S.A. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology 2009, 20, 455104. [Google Scholar] [CrossRef]
- Yang, T.; Yao, Q.; Cao, F.; Liu, Q.; Liu, B.; Wang, X. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: Insight into the cytotoxicity and antiangiogenesis. Int. J. Nanomed. 2016, 11, 6679–6692. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Bai, Y.; Zhou, J.; Li, L.; Na, J.; Fan, Y.; Guo, X.; Liu, H. A moisturizing chitosan-silk fibroin dressing with silver nanoparticles-adsorbed exosomes for repairing infected wounds. J. Mater. Chem. B 2020, 8, 7197–7212. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsu, W.S.; Chung, W.Y.; Ko, T.H.; Lin, J.H. Evaluation of various silver-containing dressing on infected excision wound healing study. J. Mater. Sci. Mater. Med. 2014, 25, 1375–1386. [Google Scholar] [CrossRef]
- Martin, M.E.; Reaves, D.K.; Jeffcoat, B.; Enders, J.R.; Costantini, L.M.; Yeyeodu, S.T.; Botta, D.; Kavanagh, T.J.; Fleming, J.M. Silver nanoparticles alter epithelial basement membrane integrity, cell adhesion molecule expression, and TGF-β1 secretion. Nanomedicine 2019, 21, 102070. [Google Scholar] [CrossRef]
- Toyokawa, H.; Matsui, Y.; Uhara, J.; Tsuchiya, H.; Teshima, S.; Nakanishi, H.; Kwon, A.-H.; Azuma, Y.; Nagaoka, T.; Ogawa, T.; et al. Promotive Effects of Far-Infrared Ray on Full-Thickness Skin Wound Healing in Rats. Exp. Biol. Med. 2003, 228, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, Antioxidant and Cytotoxic Activity of Silver Nanoparticles Synthesized by Leaf Extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Vrček, I.V.; Žuntar, I.; Petlevski, R.; Pavičić, I.; Dutour Sikirić, M.; Ćurlin, M.; Goessler, W. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environ. Toxicol. 2016, 31, 679–692. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Drake, P.L.; Hazelwood, K.J. Exposure-Related Health Effects of Silver and Silver Compounds: A Review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar]
- Li, J.; Zhang, B.; Chang, X.; Gan, J.; Li, W.; Niu, S.; Kong, L.; Wu, T.; Zhang, T.; Tang, M.; et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ. Pollut. 2020, 256, 113430. [Google Scholar] [CrossRef]
- Jia, D.; Sun, W. Silver nanoparticles offer a synergistic effect with fluconazole against fluconazole-resistant Candida albicans by abrogating drug efflux pumps and increasing endogenous ROS. Infect. Genet. Evol. 2021, 93, 104937. [Google Scholar] [CrossRef]
- Li, X.; Ahmad, K.Z.; He, J.; Li, H.; Wang, X.; Feng, Z.; Shen, G.; Ding, X. Silver nanoflowers coupled with low dose antibiotics enable the highly effective eradication of drug-resistant bacteria. J. Mater. Chem. B 2021, 9, 9839–9851. [Google Scholar] [CrossRef]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, a.; Kowalkowski, T.; et al. Evaluation of keratin biomaterial containing silver nanoparticles as a potential wound dressing in full-thickness skin wound model in diabetic mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Car—Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg.Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.I.N.; Sankar, S.; Kashif, P.M.; Basha, S.K.H.; Sastry, T.P. Evaluation of biomaterial containing regenerated cellulose and chitosan incorporated with silver nanoparticles. Int. J. Biol. Macromol. 2015, 72, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Abdi, S.; Moravvej, H.; Vossoughi, M. A comparative study of wound dressings loaded with silver sulfadiazine and silver nanoparticles: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 564, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Tra Thanh, N.; Ho Hieu, M.; Tran Minh Phuong, N.; Do Bui Thuan, T.; Nguyen Thi Thu, H.; Thai, V.P.; Do Minh, T.; Nguyen Dai, H.; Vo, V.T.; Nguyen Thi, H. Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater. Sci. Eng. C 2018, 91, 318–329. [Google Scholar] [CrossRef]
- Ali, W.; Shabani, V.; Linke, M.; Sayin, S.; Gebert, B.; Altinpinar, S.; Hildebrandt, M.; Gutmann, J.S.; Mayer-Gall, T. Electrical conductivity of silver nanoparticle doped carbon nanofibres measured by CS-AFM. RSC Adv. 2019, 9, 4553–4562. [Google Scholar] [CrossRef] [Green Version]
- Vasil’kov, A.; Dovnar, R.; Smotryn, S.; Iaskevich, N.; Naumkin, A. Plasmon Resonance of Silver Nanoparticles as a Method of Increasing Their Antibacterial Action. Antibiotics 2018, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Konop, M.; Kłodzińska, E.; Borowiec, J.; Laskowska, A.K.; Czuwara, J.; Konieczka, P.; Cieślik, B.; Waraksa, E.; Rudnicka, L. Application of micellar electrokinetic chromatography for detection of silver nanoparticles released from wound dressing. Electrophoresis 2019, 40, 1565–1572. [Google Scholar] [CrossRef]
- Reddy, V.N.; Nyamathulla, S.; Pahirulzaman, K.A.K.; Mokhtar, S.I.; Giribabu, N.; Pasupuleti, V.R. Gallocatechin-silver nanoparticles embedded in cotton gauze patches accelerated wound healing in diabetic rats by promoting proliferation and inhibiting apoptosis through the Wnt/β-catenin signaling pathway. PLoS ONE 2022, 17, e0268505. [Google Scholar]
- Kaur, P.; Sharma, A.K.; Nag, D.; Das, A.; Datta, S.; Ganguli, A.; Goel, V.; Rajput, S.; Chakrabarti, G.; Basu, B. Novel nano-insulin formulation modulates cytokine secretion and remodeling to accelerate diabetic wound healing. Nanomedicine 2019, 15, 47–57. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M.; Kumari, A.; Mahesh, S.; Padwad, Y.S.; Yadav, S.K. Cytocompatible Anti-microbial Dressings of Syzygium cumini Cellulose Nanocrystals Decorated with Silver Nanoparticles Accelerate Acute and Diabetic Wound Healing. Sci. Rep. 2017, 7, 10457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Li, Z.; Song, S.; Yang, K.; Liu, H.; Yang, Z.; Wang, j.; Yang, B.; Lin, Q. Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv. Funct. Mater. 2019, 29, 1901474. [Google Scholar] [CrossRef]
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An Antifouling Hydrogel Containing Silver Nanoparticles for Modulating the Therapeutic Immune Response in Chronic Wound Healing. Langmuir 2019, 35, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Birla, S.; Ingle, A.P.; Gade, A.; Ingle, P.; Golińska, P.; Rai, M. Superior in vivo Wound-Healing Activity of Mycosynthesized Silver Nanogel on Different Wound Models in Rat. Front. Microbiol. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Banna, A.H.E.; Youssef, F.S.; Elzorba, H.Y.; Soliman, A.M.; Mohamed, G.G.; Ismail, S.H.; Mousa, M.R.; Elbanna, H.A.; Osman, A.S. Evaluation of the wound healing effect of neomycin-silver nano-composite gel in rats. Int. J. Immunopathol. Pharmacol. 2022, 36, 039463202211134. [Google Scholar] [CrossRef]
- Wali, N.; Shabbir, A.; Wajid, N.; Abbas, N.; Naqvi, S.Z.H. Synergistic efficacy of colistin and silver nanoparticles impregnated human amniotic membrane in a burn wound infected rat model. Sci. Rep. 2022, 12, 6414. [Google Scholar] [CrossRef]
- Kong, C.; Chen, S.; Ge, W.; Zhao, Y.; Xu, X.; Wang, S.; Zhang, J. Riclin-Capped Silver Nanoparticles as an Antibacterial and Anti-Inflammatory Wound Dressing. Int. J. Nanomed. 2022, 17, 2629–2641. [Google Scholar] [CrossRef]
- Carvalho, C.D.S.; Bernardes, M.J.C.; Gonçalves, R.C.; Vilela, M.S.; Silva, M.V.M.D.; Oliveira, V.D.A.S.; Rocha, M.R.D.; Vinaud, M.C.; Junio, H.G.; Lino Junior, R.D.S. Treatment of experimentally induced partial-thickness burns in rats with different silver-impregnated dressings. Acta Cirúrgica Brasileira 2022, 37, e370801. [Google Scholar] [CrossRef]
- Davis, S.C.; Li, J.; Gil, J.; Valdes, J.; Solis, M.; Higa, A.; Bowler, P. The wound-healing effects of a next-generation anti-biofilm silver Hydrofiber wound dressing on deep partial-thickness wounds using a porcine model. Int. Wound J. 2018, 15, 834–839. [Google Scholar] [CrossRef]
- Metcalf, D.; Bowler, P. Biofilm delays wound healing: A review of the evidence. Burn. Trauma. 2013, 1, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Zhu, Y.; Lun, X.; Sheng, H.; Yan, A. Effects of wound dressing based on the combination of silver curcumin nanoparticles and electrospun chitosan nanofibers on wound healing. Bioeng. 2022, 13, 4328–4339. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Li, P.; Zhu, C.; Lin, Z.; Mackey, V.; Coy, D.H.; He, Q. The Wound Dressings and Their Applications in Wound Healing and Management. Health Sci. J. 2019, 13, 1–8. [Google Scholar]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini-Rev. Med. Chem. 2018, 18, 414–427. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochynska-Czyz, M.; Redkiewicz, P.; Kozlowska, H.; Matalinska, J.; Konop, M.; Kosson, P. Can keratin scaffolds be used for creating three-dimensional cell cultures? Open Med. 2020, 15, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Cuttle, L.; Naidu, S.; Mill, J.; Hoskins, W.; Das, K.; Kimble, R.M. A retrospective cohort study of ActicoatTM versus SilvazineTM in a paediatric population. Burns 2007, 33, 701–707. [Google Scholar] [CrossRef]
- Jones, S.A.; Bowler, P.G.; Walker, M.; Parsons, D. Controlling wound bioburden with a novel silver-containing HydrofiberR dressing. Wound Repair Regen. 2004, 12, 288–294. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Lim, Y.Q.; Low, C.Y.; Lee, C.T.; Marilyn, T.C.L.; Loh, H.S.; Lim, Y.P.; Lee, C.F.; Bhattamishra, S.K. Silver nanoparticles: Advanced and promising technology in diabetic wound therapy. Mater. Sci. Eng. C 2020, 112, 110925. [Google Scholar] [CrossRef]
- Meekul, J.; Chotirosniramit, A.; Himakalasa, W.; Orrapin, S.; Wongthanee, A.; Pongtam, O.; Kulprachakarn, K.; Rerkasem, K. A Randomized Controlled Trial on the Outcome in Comparing an Alginate Silver Dressing With a Conventional Treatment of a Necrotizing Fasciitis Wound. Int. J. Low. Extrem. Wounds 2017, 16, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.M.; Lee, I.J.; Woo, K.-J.; Park, B.Y. Silver-Impregnated Negative-Pressure Wound Therapy for the Treatment of Lower-Extremity Open Wounds: A Prospective Randomized Clinical Study. Adv. Ski. Wound Care 2019, 32, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, Q.; Hamblin, M.R.; Wen, X. A preliminary clinical trial comparing wet silver dressings versus wet-to-dry povidone-iodine dressings for wound healing in pemphigus vulgaris patients. Dermatol. Ther. 2021, 34, e14906. [Google Scholar] [CrossRef] [PubMed]
- Porro, A.M.; Seque, C.A.; Ferreira, M.C.C.; Enokihara, M.M.S.E.S. Pemphigus vulgaris. An. Bras. De Dermatol. 2019, 94, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Elmasry, M.; Steinvall, I.; Sjöberg, F.; Olofsson, P.; Thorfinn, J. Superiority of silver-foam over porcine xenograft dressings for treatment of scalds in children: A prospective randomised controlled trial. Burns 2019, 45, 1401–1409. [Google Scholar] [CrossRef]
- Akin, T.; Kendirci, M.; Akgun, A.E.; Cetinkaya, E.; Er, S.; Akin, M. Applying a Silver-containing Dressing to the Incision Site and Its Effect on the Development of Surgical Site Infection After Ostomy Closure: A Prospective Randomized Clinical Pilot Study. Wound Manag. Prev. 2022, 68, 34–43. [Google Scholar] [CrossRef]
- Asgari, P.; Zolfaghari, M.; Bit-Lian, Y.; Abdi, A.H.; Mohammadi, Y.; Bahramnezhad, F. Comparison of Hydrocolloid Dressings and Silver Nanoparticles in Treatment of Pressure Ulcers in Patients with Spinal Cord Injuries: A Randomized Clinical Trial. J. Caring Sci. 2022, 11, 1–6. [Google Scholar] [CrossRef]
- Berard, M.B.; Lau, F.H. Pilot study of minimally adherent silver dressings for acute surgical wounds. Health Sci. Rep. 2022, 5, e865. [Google Scholar] [CrossRef]
- Miner, S.A.; Lee, J.; Protzman, N.M.; Brigido, S.A. The effect of a silver hydrogel sheet dressing on postsurgical incision healing after foot and ankle surgery. Scars Burn. Heal. 2022, 8, 205951312211223. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Hou, S.L.; Li, X.W. Silver dressing in the management of an infant’s urachal anomaly infected with methicillin-resistant Staphylococcus aureus: A case report. World J. Clin. Cases 2022, 10, 2629–2636. [Google Scholar] [CrossRef]
- Wang, R.; Guo, Y.; Li, B.; Zheng, J.; Tang, Z.; Shu, M. Application Effect of Silver-Containing Dressings in the Repair of Chronic Refractory Wounds. Evid. Based Complement. Altern. Med. 2022, 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yao, D.; Ma, R.; Nan, S.; Lv, Y.; Zhu, Y.; Zhu, S. Effect of Silver Nanoparticles With Thermoplastic Polyurethane on Postoperative Rehabilitation of Diabetic Patients With Open Fracture of Lower Extremities. Front. Surg. 2022, 9, 954155. [Google Scholar] [CrossRef] [PubMed]
- Hurd, T.; Woodmansey, E.J.; Watkins, H.M.A. A retrospective review of the use of a nanocrystalline silver dressing in the management of open chronic wounds in the community. Int. Wound J. 2021, 18, 753–762. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=silver+nanoparticles (accessed on 1 October 2022).
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Norbury, W.; Herndon, D.N.; Tanksley, J.; Jeschke, M.G.; Finnerty, C.C. Infection in Burns. Surg. Infect. (Larchmt) 2016, 17, 250–255. [Google Scholar] [CrossRef] [PubMed]




| Antibacterial Reagent | Antibacterial Properties | Other Properties | References |
|---|---|---|---|
| AgNPs | gram-positive, gram-negative bacteria | Anti-angiogenic, Anti-inflammatory, Anti-oxidative | [8,10] |
| ZnO/CuO | gram-positive, gram-negative bacteria | Anti-tumor, anti-oxidative Anti-inflammatory | [15,16] |
| AuNP | gram-positive, gram-negative bacteria | Anti-inflammatory | [17] |
| Antimicrobial peptides | gram-positive, gram-negative bacteria | Anti-fungal, anti-viral, anti-parasitic, anti-tumor | [18] |
| Herbs | gram-positive, gram-negative bacteria | Anti-inflammatory, promoting angiogenesis | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybka, M.; Mazurek, Ł.; Konop, M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice. Life 2023, 13, 69. https://doi.org/10.3390/life13010069
Rybka M, Mazurek Ł, Konop M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice. Life. 2023; 13(1):69. https://doi.org/10.3390/life13010069
Chicago/Turabian StyleRybka, Mateusz, Łukasz Mazurek, and Marek Konop. 2023. "Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice" Life 13, no. 1: 69. https://doi.org/10.3390/life13010069
APA StyleRybka, M., Mazurek, Ł., & Konop, M. (2023). Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice. Life, 13(1), 69. https://doi.org/10.3390/life13010069