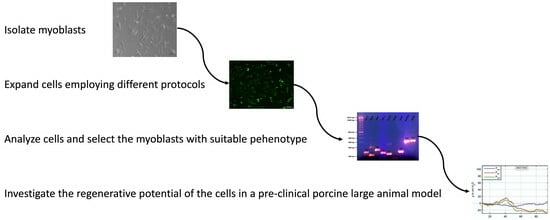
Production of Proliferation- and Differentiation-Competent Porcine Myoblasts for Preclinical Studies in a Porcine Large Animal Model of Muscular Insufficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Swine Muscle-Derived Cells and Cell Culture Procedures
2.2. Evaluation of Gene Expression on Transcript Levels
2.3. Protein Detection by Immunofluorescence
2.4. Flow Cytometry
2.5. Efficacy of Myoblast Therapy in a Preclinical Animal Study
2.6. Statistics
3. Results
3.1. Proliferation and Morphology of Swine Myoblasts
3.2. Expression of Transcripts Encoding Myogenic Markers
3.3. Detection of CD56 Using Flow Cytometry
3.4. Expression of Desmin in Swine Myoblasts
3.5. Myogenic Differentiation to Generate Myotubes
3.6. Myoblast Therapy of a Deficient Sphincter Muscle in the Animal Model of Incontinence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broome, B.A. The impact of urinary incontinence on self-efficacy and quality of life. Health Qual. Life Outcomes 2003, 1, 35. [Google Scholar] [CrossRef]
- Fultz, N.H.; Herzog, A.R. Self-reported social and emotional impact of urinary incontinence. J. Am. Geriatr. Soc. 2001, 49, 892–899. [Google Scholar] [CrossRef]
- Subak, L.L.; Brubaker, L.; Chai, T.C.; Creasman, J.M.; Diokno, A.C.; Goode, P.S.; Kraus, S.R.; Kusek, J.W.; Leng, W.W.; Lukacz, E.S.; et al. High costs of urinary incontinence among women electing surgery to treat stress incontinence. Obstet. Gynecol. 2008, 111, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Delancey, J.O. Why do women have stress urinary incontinence? Neurourol. Urodyn. 2010, 29 (Suppl. S1), S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Markland, A.D.; Goode, P.S.; Redden, D.T.; Borrud, L.G.; Burgio, K.L. Prevalence of urinary incontinence in men: Results from the national health and nutrition examination survey. J. Urol. 2010, 184, 1022–1027. [Google Scholar] [CrossRef]
- Ptak, M.; Brodowska, A.; Ciećwież, S.; Rotter, I. Quality of Life in Women with Stage 1 Stress Urinary Incontinence after Application of Conservative Treatment—A Randomized Trial. Int. J. Environ. Res. Public Health 2017, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Hillary, C.J.; Roman, S.; MacNeil, S.; Aicher, W.K.; Stenzl, A.; Chapple, C.R. Regenerative medicine and injection therapies in stress urinary incontinence. Nat. Rev. Urol. 2020, 17, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Harland, N.; Walz, S.; Eberli, D.; Schmid, F.A.; Aicher, W.K.; Stenzl, A.; Amend, B. Stress Urinary Incontinence: An Unsolved Clinical Challenge. Biomedicines 2023, 11, 2486. [Google Scholar] [CrossRef]
- Aicher, W.K.; Hart, M.L.; Stallkamp, J.; Klunder, M.; Ederer, M.; Sawodny, O.; Vaegler, M.; Amend, B.; Sievert, K.D.; Stenzl, A. Towards a Treatment of Stress Urinary Incontinence: Application of Mesenchymal Stromal Cells for Regeneration of the Sphincter Muscle. J. Clin. Med. 2014, 3, 197–215. [Google Scholar] [CrossRef]
- Schmid, F.A.; Williams, J.K.; Kessler, T.M.; Stenzl, A.; Aicher, W.K.; Andersson, K.E.; Eberli, D. Treatment of Stress Urinary Incontinence with Muscle Stem Cells and Stem Cell Components: Chances, Challenges and Future Prospects. Int. J. Mol. Sci. 2021, 22, 3981. [Google Scholar] [CrossRef]
- Peters, K.M.; Dmochowski, R.R.; Carr, L.K.; Robert, M.; Kaufman, M.R.; Sirls, L.T.; Herschorn, S.; Birch, C.; Kultgen, P.L.; Chancellor, M.B. Autologous Muscle Derived Cells for Treatment of Stress Urinary Incontinence in Women. J. Urol. 2014, 192, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Pokrywczynska, M.; Adamowicz, J.; Czapiewska, M.; Balcerczyk, D.; Jundzill, A.; Nowacki, M.; Petros, P.; Drewa, T. Targeted therapy for stress urinary incontinence: A systematic review based on clinical trials. Expert. Opin. Biol. Ther. 2016, 16, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, A.W.; Mouritsen, J.M.; Jensen, L.P.; Bødker, N.; Holst, A.W.; Pennisi, C.P.; Ehlers, L. Cell-based therapy for the treatment of female stress urinary incontinence: An early cost–effectiveness analysis. Regen. Med. 2018, 13, 321–330. [Google Scholar] [CrossRef]
- Alarcin, E.; Bal-Öztürk, A.; Avci, H.; Ghorbanpoor, H.; Dogan Guzel, F.; Akpek, A.; Yesiltas, G.; Canak-Ipek, T.; Avci-Adali, M. Current Strategies for the Regeneration of Skeletal Muscle Tissue. Int. J. Mol. Sci. 2021, 22, 5929. [Google Scholar] [CrossRef]
- Fang, J.; Sia, J.; Soto, J.; Wang, P.; Li, L.K.; Hsueh, Y.-Y.; Sun, R.; Faull, K.F.; Tidball, J.G.; Li, S. Skeletal muscle regeneration via the chemical induction and expansion of myogenic stem cells in situ or in vitro. Nat. Biomed. Eng. 2021, 5, 864–879. [Google Scholar] [CrossRef]
- Kelp, A.; Albrecht, A.; Amend, B.; Klunder, M.; Rapp, P.; Sawodny, O.; Stenzl, A.; Aicher, W.K. Establishing and monitoring of urethral sphincter deficiency in a large animal model. World J. Urol. 2017, 35, 1977–1986. [Google Scholar] [CrossRef]
- Metzger, K.; Tuchscherer, A.; Palin, M.F.; Ponsuksili, S.; Kalbe, C. Establishment and validation of cell pools using primary muscle cells derived from satellite cells of pig skeletal muscle. In Vitro Cell Dev. Biol. Anim. 2020, 56, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, F.; Liu, Y.; Li, S.; Zhou, G.; Hu, P. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discov. 2017, 3, 17003. [Google Scholar] [CrossRef]
- Brun, J.; Lutz, K.A.; Neumayer, K.M.; Klein, G.; Seeger, T.; Uynuk-Ool, T.; Worgotter, K.; Schmid, S.; Kraushaar, U.; Guenther, E.; et al. Smooth Muscle-Like Cells Generated from Human Mesenchymal Stromal Cells Display Marker Gene Expression and Electrophysiological Competence Comparable to Bladder Smooth Muscle Cells. PLoS ONE 2015, 10, e0145153. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.; Morrison, T.; Herrmann, M.; Wittwer, C. Quantitative PCR by continuous fluorescence monitoring of a double strand dna specific binding dye. Biochemica 1998, 2, 8–11. [Google Scholar]
- Kobayashi-Kinoshita, S.; Yamakoshi, Y.; Onuma, K.; Yamamoto, R.; Asada, Y. TGF-β1 autocrine signalling and enamel matrix components. Sci. Rep. 2016, 6, 33644. [Google Scholar] [CrossRef]
- Kalbe, C.; Mau, M.; Rehfeldt, C. Developmental changes and the impact of isoflavones on mRNA expression of IGF-I receptor, EGF receptor and related growth factors in porcine skeletal muscle cell cultures. Growth Horm. IGF Res. 2008, 18, 424–433. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Luo, J.; He, J.; Zheng, P.; Yu, J. Leucine promotes differentiation of porcine myoblasts through the protein kinase B (Akt)/Forkhead box O1 signalling pathway. Br. J. Nutr. 2018, 119, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Knoll, J.; Harland, N.; Amend, B.; Enderle, M.D.; Linzenbold, W.; Abruzzese, T.; Kalbe, C.; Kemter, E.; Wolf, E.; et al. Replacing Needle Injection by a Novel Waterjet Technology Grants Improved Muscle Cell Delivery in Target Tissues. Cell Transplant. 2022, 31, 9636897221080943. [Google Scholar] [CrossRef] [PubMed]
- Maak, S.; Wicke, M.; Swalve, H.H. Analysis of gene expression in specific muscle of swine and turkey. Arch. Tierz. 2005, 48, 135–140. [Google Scholar]
- Pilz, G.A.; Braun, J.; Ulrich, C.; Felka, T.; Warstat, K.; Ruh, M.; Schewe, B.; Abele, H.; Larbi, A.; Aicher, W.K. Human mesenchymal stromal cells express CD14 cross-reactive epitopes. Cytom. A 2011, 79, 635–645. [Google Scholar] [CrossRef]
- Robinson, J.P. Current Protocols in Cytometry; John Wiley and Sons, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Amend, B.; Kelp, A.; Vaegler, M.; Klunder, M.; Frajs, V.; Klein, G.; Sievert, K.D.; Sawodny, O.; Stenzl, A.; Aicher, W.K. Precise injection of human mesenchymal stromal cells in the urethral sphincter complex of Gottingen minipigs without unspecific bulking effects. Neurourol. Urodyn. 2017, 36, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Horikawa, I.; Harris, C. Cellular Senescence: Mechanisms, Morphology, and Mouse Models. Vet. Pathol. 2020, 57, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Sinanan, A.C.; Hunt, N.P.; Lewis, M.P. Human adult craniofacial muscle-derived cells: Neural-cell adhesion-molecule (NCAM; CD56)-expressing cells appear to contain multipotential stem cells. Biotechnol Appl Biochem. 2004, 40, 25–34. [Google Scholar] [CrossRef]
- Capkovic, K.L.; Stevenson, S.; Johnson, M.C.; Thelen, J.J.; Cornelison, D.D. Neural cell adhesion molecule (NCAM) marks adult myogenic cells committed to differentiation. Exp. Cell Res. 2008, 314, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Pisani, D.F.; Clement, N.; Loubat, A.; Plaisant, M.; Sacconi, S.; Kurzenne, J.Y.; Desnuelle, C.; Dani, C.; Dechesne, C.A. Hierarchization of myogenic and adipogenic progenitors within human skeletal muscle. Stem Cells 2010, 28, 2182–2194. [Google Scholar] [CrossRef]
- Capetanaki, Y.; Milner, D.J.; Weitzer, G. Desmin in muscle formation and maintenance: Knockouts and consequences. Cell Struct. Funct. 1997, 22, 103–116. [Google Scholar] [CrossRef]
- Hnia, K.; Ramspacher, C.; Vermot, J.; Laporte, J. Desmin in muscle and associated diseases: Beyond the structural function. Cell Tissue Res. 2015, 360, 591–608. [Google Scholar] [CrossRef]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Pajalunga, D.; Crescenzi, M. Restoring the Cell Cycle and Proliferation Competence in Terminally Differentiated Skeletal Muscle Myotubes. Cells 2021, 10, 2753. [Google Scholar] [CrossRef]
- Hart, M.L.; Izeta, A.; Herrera-Imbroda, B.; Amend, B.; Brinchmann, J.E. Cell Therapy for Stress Urinary Incontinence. Tissue Eng. Part B Rev. 2015, 21, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Imbroda, B.; Lara, M.F.; Izeta, A.; Sievert, K.D.; Hart, M.L. Stress urinary incontinence animal models as a tool to study cell-based regenerative therapies targeting the urethral sphincter. Adv. Drug Deliv. Rev. 2015, 82–83, 106–116. [Google Scholar] [CrossRef]
- Gotoh, M.; Yamamoto, T.; Shimizu, S.; Matsukawa, Y.; Kato, M.; Majima, T.; Takai, S.; Funahashi, Y.; Toriyama, K. Treatment of male stress urinary incontinence using autologous adipose-derived regenerative cells: Long-term efficacy and safety. Int. J. Urol. 2019, 26, 400–405. [Google Scholar] [CrossRef]
- Blaganje, M.; Lukanović, A. Intrasphincteric autologous myoblast injections with electrical stimulation for stress urinary incontinence. Int. J. Gynaecol. Obstet. 2012, 117, 164–167. [Google Scholar] [CrossRef]
- Aragón, I.M.; Imbroda, B.H.; Lara, M.F. Cell Therapy Clinical Trials for Stress Urinary Incontinence: Current Status and Perspectives. Int. J. Med. Sci. 2018, 15, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Mitterberger, M.; Pinggera, G.M.; Marksteiner, R.; Margreiter, E.; Plattner, R.; Klima, G.; Strasser, H. Functional and histological changes after myoblast injections in the porcine rhabdosphincter. Eur. Urol. 2007, 52, 1736–1743. [Google Scholar] [CrossRef]
- Burdzinska, A.; Crayton, R.; Dybowski, B.; Idziak, M.; Gala, K.; Radziszewski, P.; Paczek, L. The effect of endoscopic administration of autologous porcine muscle-derived cells into the urethral sphincter. Urology 2013, 82, 743.e1–743.e8. [Google Scholar] [CrossRef]
- Burdzinska, A.; Dybowski, B.; Zarychta-Wisniewska, W.; Kulesza, A.; Zagozdzon, R.; Gajewski, Z.; Paczek, L. The Anatomy of Caprine Female Urethra and Characteristics of Muscle and Bone Marrow Derived Caprine Cells for Autologous Cell Therapy Testing. Anat. Rec. 2017, 300, 577–588. [Google Scholar] [CrossRef]
- Burdzinska, A.; Dybowski, B.; Zarychta-Wisniewska, W.; Kulesza, A.; Butrym, M.; Zagozdzon, R.; Graczyk-Jarzynka, A.; Radziszewski, P.; Gajewski, Z.; Paczek, L. Intraurethral co-transplantation of bone marrow mesenchymal stem cells and muscle-derived cells improves the urethral closure. Stem Cell Res. Ther. 2018, 9, 239. [Google Scholar] [CrossRef]
- Jankowski, R.J.; Deasy, B.M.; Huard, J. Muscle-derived stem cells. Gene Ther. 2002, 9, 642–647. [Google Scholar] [CrossRef]
- Mau, M.; Oksbjerg, N.; Rehfeldt, C. Establishment and conditions for growth and differentiation of a myoblast cell line derived from the semimembranosus muscle of newborn piglets. In Vitro Cell. Dev. Biol.-Anim. 2008, 44, 1–5. [Google Scholar] [CrossRef]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.; Ide, C.; Nabeshima, Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yoshida, S.; Koishi, K.; Masuda, K.; Nabeshima, Y. Cell heterogeneity upon myogenic differentiation: Down-regulation of MyoD and Myf-5 generates ‘reserve cells’. J. Cell Sci. 1998, 111 Pt 6, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Hannon, K.; Kudla, A.J.; McAvoy, M.J.; Clase, K.L.; Olwin, B.B. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J. Cell Biol. 1996, 132, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.A.; Marquardt, L.M.; Heilshorn, S.C. The Diverse Roles of Hydrogel Mechanics in Injectable Stem Cell Transplantation. Curr. Opin. Chem. Eng. 2017, 15, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sasca, D.; Szybinski, J.; Schuler, A.; Shah, V.; Heidelberger, J.; Haehnel, P.S.; Dolnik, A.; Kriege, O.; Fehr, E.M.; Gebhardt, W.H.; et al. NCAM1 (CD56) promotes leukemogenesis and confers drug resistance in AML. Blood 2019, 133, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, C.J.; Scott, C.A.; Laritsky, E.; Baker, M.S.; MacKay, H.; Duryea, J.D.; Kessler, N.J.; Hellenthal, G.; Wood, A.C.; Hodges, K.R.; et al. A genomic atlas of systemic interindividual epigenetic variation in humans. Genome Biol. 2019, 20, 105. [Google Scholar] [CrossRef]
- Vinarov, A.; Atala, A.; Yoo, J.; Slusarenco, R.; Zhumataev, M.; Zhito, A.; Butnaru, D. Cell therapy for stress urinary incontinence: Present-day frontiers. J. Tissue Eng. Regen. Med. 2018, 12, e1108–e1121. [Google Scholar] [CrossRef]
- Whiting, D.; Hamdoon, M.; Sriprasad, S. Stem cell therapy for stress urinary incontinence. J. Clin. Urol. 2020, 13, 62–69. [Google Scholar] [CrossRef]








| Gen | Forward Sequence | Reverse Sequence | Accession No. | Reference | Size |
|---|---|---|---|---|---|
| GAPDH | CCATCACCATCTTCCAGGAG | ACAGTCTTCTGGGTGGCAGT | NM_001206359.1 | [21]} | 346 |
| b2MG | ACGGAAAGCCAAATTACCTGAACTG | TCTGTGATGCCGGTTAGTGGTCT | NM_213978.1 # | [22] | 261 |
| MyoG | CGCCATCCAGTACATCGAG | TGTGGGAACTGCATTCACTG | NM_001012406.1 | [23] | 125 |
| Pax7 | AGATCGCAGCAGGGGTAAAG | GACCCCACCAAGCTGATTGA | XM_021095458.1 | Primerblast | 209 |
| Myl1 | CTCTCAAGATCAAGCACTGCG | GCAGACACTTGGTTTGTGTGG | NM_214374.2 | [24] | 198 |
| Myf5 | GCTGCTGAGGGAACAGGTGGA | CTGCTGTTCTTTCGGGACCAGAC | NM_001278775.1 | [25] | 135 |
| MSTN | CCCGTCAAGACTCCTACAACA | CACATCAATGCTCTGCCAA | NM_005259.3 | [25] | 141 |
| ACT | CGGGCAGGTCATCACCATC | CGTGTTGGCGTAGAGGTCCTT | XM_005670976.2 ## | [25] | 160 |
| MYH1 | CCAGGGAGAGATGGAGGACA | TCAAGTTCACGTACCCTGGC | NM_001104951.2 | Primerblast | 258 |
| Des | ACACCTCAAGGATGAGATGGC | CAGGGCTTGTTTCTCGGAAG | NM_001001535.1 | [24] | 176 |
| Myf6 | AGTGGCCAAGTGTTTCGGATC | CGCGAGTTATTTCTCCCCCA | NM_001244672.1 | Primerblast | 179 |
| ACTA1 | ACCCGACGCCATGTGTGA | GTCGCCCACGTAGGAATCTT | NM_001167795.1 | Primerblast | 184 |
| MyoD1 | CACTACAGCGGTGACTCAGACGCA | GACCGGGGTCGCTGGGCGCCTCGCT | NM_001002824.1 | [25] | 145 |
| Gene | p-Value |
|---|---|
| Pax7 | 0.001 |
| MyoD1 | <0.001 |
| Myf5 | 0.005 |
| MyoG | 0.017 |
| Myf6 | 0.019 |
| Des | 0.005 |
| Gene | p-Value |
|---|---|
| Pax7 | 0.184 |
| Myh1 | 0.158 |
| MyoD1 | 0.006 |
| Myf5 | 0.028 |
| MyoG | 0.017 |
| Myf6 | 0.045 |
| Des | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoll, J.; Amend, B.; Abruzzese, T.; Harland, N.; Stenzl, A.; Aicher, W.K. Production of Proliferation- and Differentiation-Competent Porcine Myoblasts for Preclinical Studies in a Porcine Large Animal Model of Muscular Insufficiency. Life 2024, 14, 212. https://doi.org/10.3390/life14020212
Knoll J, Amend B, Abruzzese T, Harland N, Stenzl A, Aicher WK. Production of Proliferation- and Differentiation-Competent Porcine Myoblasts for Preclinical Studies in a Porcine Large Animal Model of Muscular Insufficiency. Life. 2024; 14(2):212. https://doi.org/10.3390/life14020212
Chicago/Turabian StyleKnoll, Jasmin, Bastian Amend, Tanja Abruzzese, Niklas Harland, Arnulf Stenzl, and Wilhelm K. Aicher. 2024. "Production of Proliferation- and Differentiation-Competent Porcine Myoblasts for Preclinical Studies in a Porcine Large Animal Model of Muscular Insufficiency" Life 14, no. 2: 212. https://doi.org/10.3390/life14020212
APA StyleKnoll, J., Amend, B., Abruzzese, T., Harland, N., Stenzl, A., & Aicher, W. K. (2024). Production of Proliferation- and Differentiation-Competent Porcine Myoblasts for Preclinical Studies in a Porcine Large Animal Model of Muscular Insufficiency. Life, 14(2), 212. https://doi.org/10.3390/life14020212